|
 Home:
Educational
Supplement: Section 9
Home:
Educational
Supplement: Section 9
TRIALS OF
NEOADJUVANT ENDOCRINE THERAPY
We've been
involved in a number of randomized neoadjuvant trials. The Impact
study is in a neoadjuvant setting, the same therapies being evaluated
in the ATAC adjuvant trial. From our prior work, we know that anastrozole
and tamoxifen have different biological effects on tumors. Anastrozole
switches off proliferation. If you look at the tumor before starting
anastrozole and then three months later, in about half of the patients
the actual histologic grade changes - it downgrades the tumor. But
the reason it downgrades is because proliferation is turned off.
If you look
at tamoxifen, about half of the patients will also downgrade, but
the downgrade is completely different. What you see is an increase
in glandular differentiation. Tamoxifen also tends to induce the
progesterone receptor, while anastrozole switches the PR off completely.
These observations
fit with the fact that different clinical activities have been seen
with aromatase inhibitors, which are actually more effective than
tamoxifen. So, we're starting to understand that there's not one
pathway for endocrine therapies. And, of course, if they act by
different mechanisms, then combinations - like in ATAC - could be
more effective than one agent alone. You can get a very good idea
of what's happening in tumors by looking at biological markers rather
than clinical endpoints.
In these
studies we've been taking biopsies at 10 to 14 days and looking
at what effect drugs have on proliferation, cell death and a variety
of genetic markers to see if we can start to predict very early
on whether the patient is going to get a benefit from a drug. And
that is potentially a very useful tool, because a lot of patients
wait 10 to 14 days (at least in the U.K.) from the time of diagnosis
to the time of surgery.
If you got
a core biopsy at the time of diagnosis and you put a patient on
a drug and then you operate on them and you see that that drug has
done something to the tumor and you know that that change is associated
with long-term benefit for a patient, then you have a potential
method to individualize treatment.
-J
Michael Dixon, MD
| PHASE
II PILOT STUDY OF CDNA MICROARRAY AS A MEASURE OF TUMOR RESPONSE
TO NEOADJUVANT DOCETAXEL AND CAPECITABINE FOLLOWED BY SURGERY
AND ADJUVANT DOXORUBICIN AND CYCLOPHOSPHAMIDE IN PATIENTS WITH
STAGE II OR III BREAST CANCER Protocol
PROTOCOL
ID: NCI-00-C-0149
PROJECTED
ACCRUAL: A
total of 18-36 patients will be accrued within 18 months.
|
|
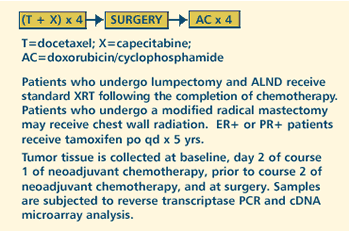
OBJECTIVES
- Evaluate the feasibility of using cDNA microarray as a
measure of a tumor's biological response to neoadjuvant
docetaxel and capecitabine followed by surgery and adjuvant
doxorubicin and cyclophosphamide by characterizing the cDNA
expression patterns before and after chemotherapy in patients
with stage II or III breast cancer.
- Determine the toxicities of this regimen in these patients.
- Determine the clinical and pathologic response rate.
PARTICIPATION
CRITERIA
- Hormone receptor status: Receptor status known
- Histologically or cytologically confirmed stage II or
III breast cancer (Tumor size greater than 2 cm)
- Prior biopsy allowed if adequate tissue remains for 2nd
biopsy
STUDY
CONTACT
JoAnne
Zujewski, Chair, Ph: 301-402-0985 Center for Cancer Research
Medicine Branch Bethesda, Maryland
|
| PHASE
I PILOT STUDY OF PREOPERATIVE TRASTUZUMAB (HERCEPTIN) AND PACLITAXEL
FOLLOWED BY POSTOPERATIVE DOXORUBICIN AND CYCLOPHOSPHAMIDE IN
WOMEN WITH LOCALLY ADVANCED BREAST CANCER WITH HER2 OVEREXPRESSION
Protocol
PROTOCOL
IDS: NYU-9901,
NCI-G00-1905, GENENTECH-NYU-9901
PROJECTED
ACCRUAL: A
total of 15 patients will be accrued for this study.
|
OBJECTIVES
- Determine the safety and toxicity of preoperative trastuzumab
and paclitaxel followed by postoperative doxorubicin and
cyclophosphamide in women with locally advanced breast cancer
with HER2 overexpression.
- Determine tumor response in these patients.
- Assess the effect of this regimen on tumor histology and
the potential molecular determinants of response in these
patients.
PARTICIPATION
CRITERIA
- Age 18 and over
- Palpable primary breast cancer at least 3 cm (T2 at least
3 cm, T3-T4, any N), no distant metastasis (M0)
- HER2 overexpression of 2+ or 3+ by IHC on core biopsy
specimen using the Dako Hercep Test
- Hormone receptor status known
- No prior chemotherapy
PROTOCOL
Patients
receive trastuzumab followed by paclitaxel on day 1. q 7 days
x 10 courses in the absence of disease progression or unacceptable
toxicity. Modified radical mastectomy or a lumpectomy with
AND. Beginning 14 days after surgery, patients receive doxorubicin
and cyclophosphamide over 15-30 minutes on day 1. Treatment
repeats q 21 days x 4 courses. After chemotherapy, patients
with hormone receptor-positive disease receive tamoxifen x
5 years.
STUDY
CONTACT
Matthew
D. Volmi, Chair, Ph: 212-263-6485 NYU School of Medicine's
Kaplan Comprehensive Cancer Center New York, New York
|
Page
3of 3
Previous
| Select Publications
Top
Additional
Sections:
1 | 2
| 3 | 4
| 5 | 6
| 7 | 8
| 9 | 10
| 11 | 12
|
|
|

