|
 Home:
Educational
Supplement: Section 7
Home:
Educational
Supplement: Section 7
Section 7
Optimal
use of adjuvant chemotherapy
OVERVIEW
The International
Breast Cancer Overview clearly demonstrates that adjuvant chemotherapy
has a significant impact on disease-free and overall survival, particularly
in premenopausal women. A key current issue in ongoing trials in
optimizing treatment is defining the role of adjuvant taxanes, including
selection of patients, choice of paclitaxel versus docetaxel and
optimal scheduling.
The NIH Consensus
statement noted the small but statistically significant advantage
conferred by anthracycline-containing regimens, but concluded that
the optimal anthracycline-containing regimen has not been identified.
Current data on taxanes was considered inconclusive. The statement
further noted that chemotherapy is generally offered to women with
tumors over one centimeter, but in women with node-negative tumors
under a centimeter, treatment should be individualized, as subsets
of these patients have an excellent prognosis. For the first time,
a major cooperative group trial will specifically focus on chemotherapy
for elderly patients, evaluating the orally administered fluoropyrimidine,
capecitabine, with the hope that efficacy will be comparable to
conventional regimens, but with less toxicity.
NIH
STATEMENT
...Available
data indicate that adjuvant chemotherapy regimens that include an
anthracycline result in a small but statistically significant improvement
in survival compared to nonanthracycline-containing programs.
...Randomized
trials have demonstrated threshold dose effects for two of the most
active chemotherapeutic agents, doxorubicin (A) and cyclophosphamide
(C). These two drugs are frequently administered together (AC) and
appear to result in a comparable survival outcome, whether given
preoperatively or postoperatively. However, AC has not been compared
to cyclophosphamide/doxorubicin/5-fluorouracil (CAF) or cyclophosphamide/epirubicin/5-fluorouracil
(CEF).
On the basis
of available data, it is accepted practice to offer cytotoxic chemotherapy
to most women with lymph node metastases or with primary breast
cancers larger than 1 cm in diameter (both node-negative and node-positive).
For women with node-negative cancers less than 1 cm in diameter,
the decision to consider chemotherapy should be individualized.
Taxanes (docetaxel,
paclitaxel) have recently been demonstrated to be among the most
active agents in the treatment of metastatic breast cancer. As a
result, several studies have explored the clinical utility of adding
these drugs to standard doxorubicin/cyclophosphamide treatment programs
in the adjuvant treatment of node-positive, localized breast cancer.
Although
a number of such trials have completed accrual and others remain
in progress, currently available data are inconclusive and do not
permit definitive recommendations regarding the impact of taxanes
on either relapse-free or overall survival. There is no evidence
to support the use of taxanes in node-negative breast cancer outside
the setting of a clinical trial.
-NIH
Consensus Conference 2000
Final Statement. Full-Text
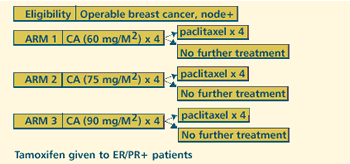
NSABP
B-28: PHASE III RANDOMIZED STUDY OF PACLITAXEL VS NO FURTHER CHEMOTHERAPY
FOLLOWING DOXORUBICIN/ CYCLOPHOSPHAMIDE FOR RESECTED NODE-POSITIVE
BREAST CANCER (Closed to Accrual) Protocol

Mamounas
EP. Evaluating the use of paclitaxel following doxorubicin/ cyclophosphamide
in patients with breast cancer and positive axillary nodes.
NIH Consensus Conference on Early Breast Cancer, 2000. Abstract
CLB-9344;
INT-0148: PHASE III RANDOMIZED STUDY OF ADJUVANT CA (CYCLOPHOSPHAMIDE/DOXORUBICIN)
COMPARING STANDARD VS INTERMEDIATE VS HIGH-DOSE DOXORUBICIN WITH
VS WITHOUT SUBSEQUENT PACLITAXEL IN WOMEN WITH NODE-POSITIVE BREAST
CANCER (Closed to Accrual) Protocol
Henderson,
IC et al. Adjuvant chemotheraphy: Taxanes — the “pro”
position. NIH Conference on Early Breast Cancer, 2000. Abstract
ADJUVANT
TAXANES
The addition
of four cycles of paclitaxel after the completion of a standard
course of CA substantially improves disease-free and overall survival
of patients with early breast cancer. In light of more recent smaller
but nonconfounded studies that demonstrated an advantage from adding
four cycles of single agent taxane to four cycles of a doxorubicin-containing
regimen, it is highly unlikely that all of the benefit for patients
on the paclitaxel arm of this study is due simply to the longer
duration of treatment in that arm of the trial.
-I
Craig Henderson, MD et al. NIH
Consensus Conference 2000. Abstract
It
is necessary to wait for future results of ongoing trials before
pronouncing judgment on the value of taxanes in the adjuvant setting.
It is also necessary to better define the population most likely
to benefit from therapies of longer duration, intensification and
multiple regimens. It no longer is reasonable to judge all breast
cancer patients as having equal probability of benefit from a given
therapy. That was a paradigm that worked well when adjuvant chemotherapy
for breast cancer was in its infancy and little was known about
the molecular heterogeneity of breast cancer. It is now of critical
importance to design trials with the aid of molecular tumor profiles
with potential predictive value to prospectively identify the subgroup
most likely to benefit from the addition to therapy of taxanes and
other new drugs.
-Martine
J Piccart, MD, PhD et al. NIH
Consensus Conference 2000. Abstract
Both the
NIH and St Gallen Consensus Conferences expressed a lack of enthusiasm
for endorsing the addition of the taxanes as a routine in node-positive
patients, because the data were not mature enough to make that decision
at this time. One of the more alarming things about increasing the
duration of chemotherapy is the issue of long-term cognitive problems,
and we all see the patient who clearly is a different person mentally
after chemotherapy. Certainly there are many possible causes for
this - above and beyond cognitive deficits induced by chemotherapy
- including the psychological effects of a cancer diagnosis with
stress and depression. But it would be very nice to have some strong
data on cognition, because that could be a real problem for a one-
or two-percent survival tradeoff.
-Monica
Morrow, MD
ADJUVANT
CHEMOTHERAPY FOR EARLY BREAST CANCER
The seminal
studies that demonstrated that long-term chemotherapy could have
an important survival advantage emerged from the National Surgical
Adjuvant Breast and Bowel Project, led by Professor Bernard Fisher,
and the historic trial of cyclophosphamide, methotrexate and fluorouracil
led by Dr Gianni Bonadonna in Milan. It was probably this second
trial more than any other that established the role of adjuvant
chemotherapy in the management of early breast cancer. Many subsequent
trials have attempted to fine-tune or better select the optimum
duration and combination of cytotoxic drugs.
-Michael
Baum, ChM, FRCS; Joan Houghton BSc 1999;319:568-571 Full-Text
ADJUVANT
THERAPY FOR LOW-RISK PATIENTS
Sometimes
we neglect using some fairly well-established, reproducible prognostic
factors. The SEER data and the American College of Surgeons' National
Cancer Database cannot reliably identify a subset of node-negative
patients with tumors under a centimeter with a long-term survival
of less than 90 percent.
There are
also very good data showing that patients with histologic grade
one tumors that are 1.1 to 2 centimeters have a survival that's
greater than 90 percent. Patients with grade one tumors are overwhelmingly
ER-positive, so endocrine therapy is certainly a reasonable choice
there. But the added absolute benefit of chemotherapy in this subset
is extremely small, and sometimes that is not conveyed to women
in a way that they can understand.
Another group
is the special histologic subtypes, most notably tubular carcinoma
but also mucinous carcinoma. Even a two to three centimeter tubular
cancer has an outstanding prognosis, and, again, these are all receptor-positive.
So, if physicians look at old consensus guidelines and just go in
lock-step with the greater-than-1-centimeter number, they might
potentially overtreat some patients by using chemotherapy.
-Monica
Morrow, MD
CHOICE
OF ANTHRACYCLINE REGIMEN
The 1995
overview established that anthracycline-containing regimens added
benefit, but the choice of a specific regimen is also an issue.
My reading of the literature is that we were too hasty in adopting
four cycles of AC as a standard, and it was based more on convenience
than true superiority over the previous standard, which was CMF.
In fact, there are no convincing data that AC times four is superior
to CMF.
On the other
hand, if you look at the overview of randomized trials - with and
without anthracyclines - there is superiority in favor of the anthracyclines.
Most of that comes from three-drug regimens. And you can interpret
that as either the contribution of 5-fluorouracil - which is usually
the third drug - or the contribution of duration of therapy. Most
of the three-drug combinations have been administered for six cycles,
as opposed to the AC or EC regimens, which were given mostly for
four cycles. In the adjuvant setting, one should try to use what
has - at least on the basis of controlled trials - given the best
results. In this case, I think we have compromised instead of going
for the best.
I do not
have incontrovertible proof that my hypothesis is completely correct,
but I think there is more evidence on this side than on the other.
And if you read the original NSABP B-15 paper that compared AC versus
CMF, it is clear that the reason for recommending adoption of AC
is simply one of convenience.
I have stated
this in public, including at the NIH Consensus Development Conference,
although I was unsuccessful in making this sufficiently clear to
the panel to sway their opinion. The Canadians are pursuing a clinical
trial that will go to the heart of this matter by comparing FEC
versus EC/Taxol versus AC/Taxol.
-Gabriel
Hortobagyi, MD
Page
1of 3
Next Page
Top
Additional
Sections:
1 | 2
| 3 | 4
| 5 | 6
| 7 | 8
| 9 | 10
| 11 | 12
|
|

