|
 Home:
Educational
Supplement: Section 2
Home:
Educational
Supplement: Section 2
Section 2
Management
of the axilla
OVERVIEW
A series of
classic randomized trials - including NSABP B-04 - formed the basis
for level 1 and 2 axillary node dissection becoming a standard of
care for invasive breast cancer. The emergence of sentinel node
biopsy (SNB) as an initial staging procedure has led to a new generation
of trials evaluating the need for axillary dissection in women with
both pathologically negative and positive SNB.
In the United
States, NSABP B-32 is evaluating the management of patients with
negative sentinel node biopsies by randomization to either axillary
node dissection or no further axillary surgery. While many investigators
are convinced that the anatomic hypothesis of the sentinel node
has been demonstrated, one important outcome of the NSABP trial
will be to observe the accuracy and reproducibility of this procedure
with many surgeons in various clinical settings.
Another key
U.S. sentinel node trial is being conducted by the American College
of Surgeons. This groundbreaking study will evaluate whether axillary
node dissection is necessary in sentinel node-positive patients
treated with lumpectomy and breast irradiation. A key factor is
whether the additional antitumor effect of adjuvant systemic therapy
mitigates the need for axillary dissection in these women.
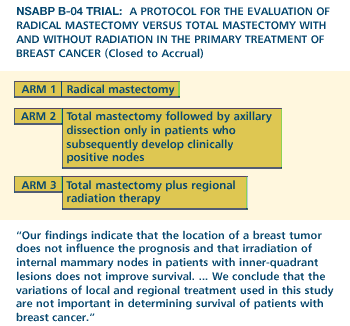
Fisher
B et al. Ten-year results of a randomized clinical trial comparing
radical mastectomy and total mastectomy with or without radiation.
N Eng J Med 1985; 312:674-681. Abstract
AXILLARY
DISSECTION FOR SNB-POSITIVE PATIENTS
For 20 years,
we have been hearing that axillary surgery is a staging procedure.
However, once you have a positive node, the patient is staged, so
does it make sense to subject her to the morbidity of an axillary
dissection? I think the answer to that right now is, yes, for several
reasons.
First, we
know that axillary dissection will maintain local control in the
axilla in 98-99 percent of patients - whether they're node-positive
or node-negative. That's very important because uncontrolled axillary
disease is extremely difficult to treat and extremely morbid. Also,
medical oncologists need to accurately estimate the risk of recurrence
to educate patients about the risks and benefits of adjuvant therapy.
To do that, in general, you need to know the number of positive
nodes.
The therapeutic
benefit of axillary dissection remains open. NSABP B-04 showed no
survival benefit, but the trial was basically a premammography era
study so the patients had larger tumors, and no adjuvant therapy
was being used at that time. Also, the sample size was not large
enough to exclude a small survival difference that today we would
think is clinically relevant.
Currently,
the standard management of a positive sentinel node is to complete
the axillary dissection, although there are individual circumstances
where that might not be appropriate. I'm proud to say that our center
has the second-highest accrual to the American College of Surgeons
trials - basically asking the same question as NSABP B-04 in a modern
setting. Without these trials, we will still be asking this question
10 years from now and making random decisions.
-Monica
Morrow, MD
ACCRUAL TO
SENTINEL NODE TRIALS
In some ways
sentinel node mapping is becoming a victim of its own success. As
surgeons realize that it is not a terrific technical feat to learn
how to do this, and as more patients become aware of it through
the Internet and other sources, it will become harder and harder
to find both patients and physicians willing to participate in these
randomized clinical trials.
-Patrick
Borgen, MD
CONTRIBUTION
OF SURGEONS TO BREAST CANCER RESEARCH
Although
other physicians are being increasingly involved in the primary
management of cancer, surgeons remain largely responsible for determining
such care and for deciding whether patients enter clinical trials.
Surgeons have not only inaugurated the use of systemic adjuvant
therapy and continue to be leaders in that effort, they have also
redefined the basis for oncologic surgical operation and, in so
doing, have contributed to a better understanding of the biology
of cancer and, consequently, its treatment.
-Bernard
Fisher, MD
| PHASE
III RANDOMIZED STUDY OF SENTINEL NODE DISSECTION WITH OR WITHOUT
CONVENTIONAL AXILLARY DISSECTION IN WOMEN WITH CLINICALLY NODE-NEGATIVE
BREAST CANCER Protocol
PROTOCOL
ID: NSABP-B-32
PROJECTED
ACCRUAL: A total of 4,000 patients (2,000 per arm) will
be accrued for this study within 4 years.
|
|

All patients
receive technetium Tc 99m sulfur colloid injected into normal
breast tissue within 1 cm of the primary tumor or biopsy cavity,
approximately 0.5-8 hours before surgery. Patients also receive
an injection of isosulfan blue dye around the tumor or biopsy
cavity after a hot spot is identified with a gamma detector.
OBJECTIVES
- Compare the long-term control of regional disease by sentinal
node resection vs sentinal node resection followed by conventional
axillary dissection in women with breast cancer who are
clinically node-negative and pathologically sentinal node-negative.
- Compare the effect of these two regimens on the overall
and disease-free survival of these patients.
- Compare the morbidity associated with these two regimens
in these patients.
- Compare the prognostic value of these two regimens in
patients who are sentinal node-negative or positive by pathology.
- Determine the potentially increased risk of systemic recurrence
in patients who are node-negative by pathology.
- Determine the technical success rate of sentinal node
dissection and the variability of technical success rate
in a broad population of surgeons.
- Determine the sensitivity of the sentinal node to determine
the presence of nodal metastases in these patients.
PARTICIPATION
CRITERIA
- Resectable invasive adenocarcinoma of the breast
- Clinically negative lymph nodes
- No positive ipsilateral axillary lymph nodes
- No prior removal of ipsilateral axillary lymph nodes
- No suspicious palpable nodes in the contralateral axilla
or palpable supraclavicular or infraclavicular nodes,
unless proven nonmalignant by biopsy
- No ulceration, erythema, infiltration of the skin or underlying
chest wall (complete fixation), peau d'orange or skin edema
of any magnitude. Tethering or dimpling of the skin or nipple
inversion allowed
- No bilateral malignancy or mass in the opposite breast
that is suspicious for malignancy, unless proven nonmalignant
by biopsy
- No diffuse tumors or multiple malignant tumors in different
quadrants of the breast
- No other prior breast malignancy except lobular carcinoma
in situ
- No breast implants
STUDY
CONTACT
David
N. Krag, Chair, Ph: 802-656-5830
National Surgical Adjuvant Breast and Bowel Project
Pittsburgh, Pennsylvania
|
Page
1of 2
Next
page
Top
Additional
Sections:
1 | 2
| 3 | 4
| 5 | 6
| 7 | 8
| 9 | 10
| 11 | 12
|
|

