|
 Home:
Educational
Supplement: Section 6
Home:
Educational
Supplement: Section 6
Section 6
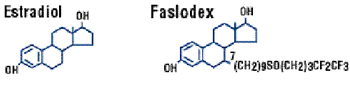
Faslodex®(fulvestrant):
An estrogen receptor downregulator
OVERVIEW
Numerous phase
III randomized clinical trials have demonstrated the efficacy of
selective estrogen receptor modulators (SERMs) in women at increased
risk for breast cancer, DCIS and invasive disease in the adjuvant
and metastatic setting. Until recently, antiestrogens had minimal
impact on patients progressing on tamoxifen, but Faslodex, a novel
agent with a mechanism distinct from SERMs, has now demonstrated
efficacy at least equivalent to anastrozole in tamoxifen-resistant
patients. Faslodex is being compared to tamoxifen for advanced disease,
and a new generation of trials will evaluate neoadjuvant and adjuvant
therapy with this unique endocrine agent.
BIOLOGIC
EFFECTS
Faslodex
is a very interesting agent. I was a co-author on one of the very
early Phase 1 studies looking at the mechanism of action. Surprisingly,
as well as it being a pure antiestrogen, Faslodex is an estrogen
receptor downregulator. It's an extraordinarily elegant molecule.
Biologically, it's very attractive, because there's no other molecule
that works as an estrogen receptor antagonist and downregulator,
and also as an antiangiogenic agent.
-Michael
Baum, ChM, FRCS
MECHANISM
OF ACTION
If you look
at the spectrum of drugs that interact with the estrogen receptor,
estrogen is on one end of the spectrum, stimulating most genes under
its control after binding to the estrogen receptor. Drugs like tamoxifen
stimulate some genes and inhibit others, depending on the tissue
and gene. At the far other end of the spectrum, drugs like Faslodex
seem to have a pure antiestrogenic profile on all genes with none
of the agonist qualities of tamoxifen.
There are
several activation domains on the estrogen receptor protein - areas
that seem to be important in activating transcription of genes.
Tamoxifen blocks only one of these. The other is still active, which
may give rise to the agonist qualities of tamoxifen. Faslodex, in
contrast, blocks all of the activation domains - both AF-1 and AF-2.
Faslodex also reduces the level of estrogen receptor in cells. In
some cases, you can't measure any estrogen receptor after exposure
to Faslodex. So, Faslodex doesn't have any agonist activity, blocks
all transcription domains and gets rid of the estrogen receptor.
-C
Kent Osborne, MD

Mode of action
of estradiol (E)
- E binds with high affinity to the estrogen receptor (ER) to
form an E-ER complex.
- The E-ER complex pairs with another E-ER complex (dimerizes)
and localizes in the cell nucleus. The two transcription activation
functions of ER, called AF1 and AF2, are both active.
- The E-ER dimer binds to estrogen-sensitive genes at the estrogen
response element (ERE).
- The dimer activates transcription of the estrogen-sensitive
gene. (AF1 and AF2 interact with coactivators to stimulate the
transcription enzyme RNA polymerase II [RNA POL II].)
Mode of action
of tamoxifen (T)
- T binds to ER with low affinity compared with E.
- The T-ER complex dimerizes and localizes in the cell nucleus,
and AF1 (but not AF2) is active.
- The T-ER dimer binds to the gene DNA at the ERE.
- Transcription of the estrogen-sensitive gene is lessened because
AF2 is inactive and coactivator binding is likewise attenuated.
AF1 activity results in the partial agonist activity of T.
Mode of action
of Faslodex (F)
- F binds to ER with affinity similar to E.
- F triggers rapid degradation of ER.
- The F-ER complex results in reduced rate of dimerization and
nuclear localization.
- There is reduced binding of F-ER to ERE on the gene resulting
in blocked transcription.
SECOND-LINE
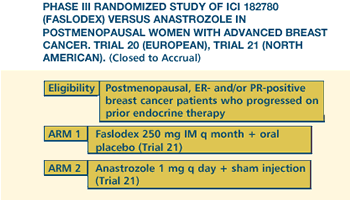
TRIALS FOR METASTATIC DISEASE: FASLODEX VERSUS ARIMIDEX
The European
trial and the American trial are a little bit different in their
structure and results. The American trial - which I think has a
better design - was a double-blind study. Patients assigned to Arimidex
received placebo injections. So, it's clearly blinded. Also, because
the patients in both groups had to come to the clinic once a month,
there was consistency with regard to the patient being seen.
The European
trial was not double-blinded. The patients on Arimidex were seen
every three months, while the patients on Faslodex were seen by
someone every month. This design has the potential to have some
bias in terms of identifying when the patient progresses. Patients
in the Faslodex group of the European trial were seen more often,
and conceivably progression would be identified a little earlier
than in an Arimidex patient.
Having said
that, the results show similar response rates between the two drugs,
but in the American trial the response duration is about twice as
long for Faslodex compared to Arimidex. We have to keep it in mind
that aromatase inhibitors are very good agents in and of themselves,
and in one of these new trials, Faslodex is at least as good as
Arimidex, and in the other we see an advantage at least in one important
parameter.
-C
Kent Osborne, MD
In terms
of efficacy, Faslodex was at least equal to treatment with a third-generation
aromatase inhibitor, which currently is our best endocrine therapy
for postmenopausal patients. There were also some hints that Faslodex
might be a bit better, mainly in the North American trial, where
the overall rates of objective response and clinical benefit were
slightly higher for Faslodex, though not statistically significant.
The duration of response in Trial 0021 was significantly better
for Faslodex and was of a magnitude that is clinically and humanly
worthwhile in the metastatic setting. These results suggest that
perhaps this agent also might give an extra boost as adjuvant therapy
which will be tested in clinical trials.
-Richard
Elledge, MD
NONBREAST
EFFECTS OF FASLODEX
This is a
completely new class of antiestrogen. It differs from tamoxifen
and other SERMS in that it increases degradation of the estrogen
receptor, resulting in dramatic reductions in this protein. It also
shuts down both AF1 and AF2 transcription functions, and it appears
not to have any estrogenic activity. This agent may be one of the
most important new developments in endocrine therapy.
The lack
of estrogen agonistic properties would clearly be beneficial in
terms of the endometrium. Thus far from available studies, Faslodex
seems to have a neutral effect on bone or lipids, but we need more
information about those systems.
Faslodex
doesn't seem to cause hot flashes, probably because it is not thought
to cross the blood-brain barrier. It is given by monthly intramuscular
injection, which is well-tolerated. Patients in the metastatic setting
don't seem to have a problem coming for the injection, because it
provides more contact with the oncology nurse. In the adjuvant setting,
if Faslodex proves to be more efficacious, I have no question that
an injection once a month will not be a deterrent to patients receiving
this agent.
We have identified
a new endocrine therapy option for postmenopausal breast cancer
patients, and it is exciting to now have a second antiestrogen,
which is clinically active in patients who become tamoxifen-resistant.
This is the first time we've ever seen an antiestrogen produce clinically
significant response rates in a randomized trial in tamoxifen-resistant
patients. That is really quite a significant event, and I think
that these studies will change the concept of antiestrogen therapy
in the same way the Arimidex-Megace studies changed concepts of
endocrine therapy of breast cancer.
-John
F Robertson, MD, FRCS
EFFECTS IN
PREMENOPAUSAL PATIENTS
There's been
some reservation about the use of Faslodex in premenopausal women,
because the large randomized trials were in postmenopausal patients.
In Trial 19 in premenopausal women about to undergo hysterectomy
for fibroids, there was no increase in side effects compared to
placebo. It was very well-tolerated. A number of biologic endpoints
were measured and were unchanged. Unlike tamoxifen, estrogen levels
did not significantly increase when Faslodex was given to premenopausal
patients, and there's no biologic reason to think that it wouldn't
be effective in these women. We used to think that tamoxifen wouldn't
be effective in premenopausal patients, but the Overview demonstrates
that tamoxifen has efficacy that's not measurably different than
in postmenopausal patients.
-Richard
Elledge, MD
INTRAMUSCULAR
INJECTION
The good
thing is that coming in every month and getting the injection is
not a big problem. It's a 5 cc injection, which for some reason
terrorized everybody in the beginning, and initially we actually
gave it in split doses. But in fact, you can give 5 cc's or 2.5
cc's twice and neither of them cause much in the way of local problems
at all. It's very well-tolerated. In the trials, we could not tell
which were Faslodex injections and which we re the placebo injections.
If this drug is move d into the adjuvant setting, that could be
more of an issue, but in the metastatic setting it doesn't seem
to be an issue for our patients at all. We've had some experience
in the adjuvant setting using another injectable over a five-year
period, and actually once you get the mechanisms in place to deliver
the injection, it doesn't seem to be a big issue.
-
Kathleen Pritchard, MD
SECONDARY
EFFECTS
There is
some data in animals suggesting that the effects of Faslodex on
bone may be neutral or even slightly positive. This may relate to
the types of estrogen receptor and tissue-specific effects. I believe
that Faslodex downregulates both alpha and beta estrogen receptor,
but there are co-regulators, and there could be a number of other
more complicated things going on that may vary from one tissue to
another. The whole area is very complex. We understand a lot about
it, but like many issues in breast cancer, the more we understand,
the more we have to ask. It's possible that a drug like Faslodex
is not a pure antiestrogen at all, but rather a complex drug that
interacts differently with receptors in bone and in some other tissues.
Certainly, in the long term, osteoporosis and heart disease are
issues that will have to be explored.
-
Kathleen Pritchard, MD
COMBINING
FASLODEX AND AN AROMATASE INHIBITOR
Conceptually,
it's a very interesting compound, and I think it's going to be an
exciting agent as we move forward. We participated in both trials
of Faslodex versus Arimidex and Faslodex versus tamoxifen. It would
be interesting to combine Faslodex with another agent with a different
mechanism of action - perhaps an aromatase inhibitor. In the past,
we had problems with tolerability when we combined endocrine agents.
Now we have more selective compounds, and the idea of combining
therapies is being revisited - for example, the ATAC trial where
Arimidex is being combined with tamoxifen as adjuvant therapy.
Preclinically
in animal models, Faslodex didn't have a uterotropic effect, so
you didn't end up with endometrial hyperplasia. If the metastatic
data look promising enough, it would be reasonable to look at that
kind of compound in the adjuvant setting, but we would also need
to evaluate the effects on the heart, bone, etc.
The monthly
intramuscular injection doesn't seem to be much of a deterrent.
For older patients where there may be a compliance question, the
injection may be a plus. Also there are some patients where reimbursement
may be more favorable for an injectable agent. Other patients may
not like the idea of an injection or coming to the doctor's office
once a month - they prefer a pill. Efficacy will be the key determining
factor though, particularly if we move to the adjuvant setting.
-
William Gradishar, MD
| PHASE
II STUDY OF FULVESTRANT (ICI 182780) IN WOMEN WITH METASTATIC
BREAST CANCER WHO HAVE FAILED AROMATASE INHIBITOR THERAPY Protocol
PROTOCOL
ID: NCCTG-N0032
PROJECTED
ACCRUAL: Approximately
41-94 patients will be accrued for this study within 10 months.
|
OBJECTIVES
- Determine the complete and partial objective response
rate and duration of response in women with metastatic breast
cancer who have failed aromatase inhibitor therapy treated
with fulvestrant.
- Determine the time to progression and overall survival.
- Determine the toxicity of this drug in these women.
PARTICIPATION
CRITERIA
- Postmenopausal women aged 18 and over
- Progressive local-regional or metastatic adenocarcinoma
- ER- and/or PR- positive or unknown with evidence of hormone
sensitivity
- Disease progression after prior 3rd generation AI
PROTOCOL
Fulvestrant
IM on day 1. Treatment repeats q 28 days in the absence of
disease progression or unacceptable toxicity. Patients followed
q 3 months x 5 years or until progression. After progression,
patients followed q 3 months x 2 years and then q 6 months
x 3 years
STUDY
CONTACT
James
N Ingle, Chair Ph: 507-284-8432
North
Central Cancer Treatment Group
|
|
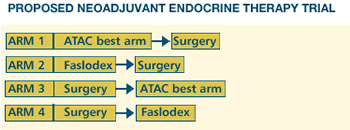
We
are looking for a trial to replace ATAC, and we are considering
evaluating conventionally timed versus perioperative endocrine
therapy. Faslodex lends itself to that because of its rapid
effects on the tumor in situ, and you can measure surrogate
markers. We would like to take the best arm of ATAC and compare
that with Faslodex in a factorial way - looking at drug against
drug and timing against timing. Hopefully, we'll have a pilot
protocol ready to go next year. This type of trial - where
you're looking at the tumor intact - can be a gold mine in
the search for the valuable surrogate markers of response.
-Michael
Baum, ChM, FRCS
|
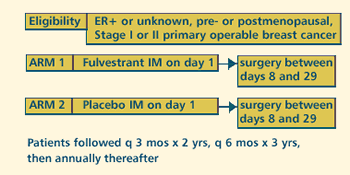
| PHASE
III RANDOMIZED NEOADJUVANT STUDY OF ICI 182780 IN WOMEN WITH
STAGE I OR II PRIMARY BREAST CANCER Protocol
PROTOCOL
ID: EORTC-10963
PROJECTED
ACCRUAL: A
total of 3,656 patients (1,828 per arm) will be accrued for
this study within 2 years.
|
|
OBJECTIVES
- Determine the inhibitory effect of ICI 182780 on the development
of metastasis, as measured by disease-free survival and
overall survival, in women with operable stage I or II primary
breast cancer.
- Determine toxicity of this regimen in these patients.
PARTICIPATION
CRITERIA
- Premenopausal or postmenopausal
- Histologically or cytologically confirmed stage I or II
primary operable breast cancer
- Surgery planned within next 1-4 weeks
- Estrogen receptor-positive or unknown
- Unknown progesterone receptor status eligible
- No known estrogen receptor-negative tumor
- At least 2 months since prior ICI 182780
- No prior XRT to primary tumor
- No other concurrent preoperative therapy for breast cancer
STUDY
CONTACT
Cornelis
J H van de Velde, Ph: 31-71-5262309
EORTC Breast Cancer Group Leiden
University Medical Center
Leiden, Netherlands
Anthony
Howell, Ph: 446-3746 ext 0161
Breast International Group Christie Hospital, NHS Trust Manchester,
England, United Kingdom
|
| The
preoperative EORTC trial evaluates one injection of Faslodex
after the diagnosis of breast cancer has been made but before
surgery. The idea is for the Faslodex injection to cover the
operative period as a potent antiestrogen that will lower estrogen
receptor levels. We want to test the hypothesis of Bernie Fisher
and others that adverse events related to metastasis occur during
the perioperative period, and hopefully we can alter that with
Faslodex. The aim is to put more than 3,000 women into this
study.
-Professor
Anthony Howell, FRCP
|
Select
Publication
Top
Additional
Sections:
1 | 2
| 3 | 4
| 5 | 6
| 7 | 8
| 9 | 10
| 11 | 12
|
|
|

