|
 Home:
Educational
Supplement: Section 9
Home:
Educational
Supplement: Section 9
| PHASE
III RANDOMIZED STUDY OF PREOPERATIVE DOXORUBICIN AND CYCLOPHOSPHAMIDE
(AC) VERSUS PREOPERATIVE AC FOLLOWED BY DOCETAXEL VERSUS PREOPERATIVE
AC AND POSTOPERATIVE DOCETAXEL IN WOMEN WITH OPERABLE CARCINOMA
OF THE BREAST (Closed to Accrual) Protocol
PROTOCOL
ID: NSABP
B-27
PROJECTED
ACCRUAL: Approximately
2,411 patients were accrued.
|
|
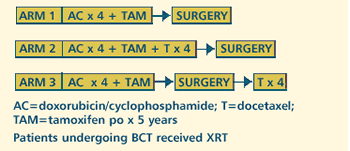
OBJECTIVES
- Compare overall and disease-free survival in patients
with operable breast cancer treated with four courses of
preoperative AC alone versus four courses of preoperative
or postoperative docetaxel following four courses of preoperative
AC .
- Evaluate whether the addition of pre operative docetaxel
to preoperative AC results in improved rates of clinical
and pathologic locoregional tumor response .
- Assess whether the addition of pre operative docetaxel
to properative AC results in improved rates of breast conservation
.
- Assess whether postoperative docetaxel improves disease-free
and overall survival in patients who receive preoperative
AC, especially in certain subgroups of patients (e.g., those
with pathologically positive nodes).
PARTICIPATION
CRITERIA
- Any age, with life expectancy of >=10 years exclusive
of cancer diagnosis
- Histologically or cytologically proven invasive breast
cancer
- <= 63 days between diagnosis and randomization
- Tumor palpable on clinical exam and confined to the breast
and ipsilateral axilla
- If clinically negative axillary nodes (N0): primary tumor
greater than 1 cm (T1c-T3)
- If clinically positive axillary nodes (N1): any size primary
tumor (T1-3)
- No N2 disease
- ER/PR-positive or negative
STUDY
CONTACT
Norman
Wolmark, Chair, Ph: 412-359-3336 National Surgical Adjuvant
Breast and Bowel Project Allegheny General Hospital, Pennsylvania
|
| PHASE
III RANDOMIZED STUDY OF NEOADJUVANT DOXORUBICIN AND CYCLOPHOSPHAMIDE
WITH OR WITHOUT FILGRASTIM (G-CSF) IN WOMEN WITH INFLAMMATORY
OR ESTROGEN RECEPTOR-NEGATIVE LOCALLY ADVANCED BREAST CANCER
Protocol
PROTOCOL
IDS: SWOG-S0012,
CTSU
PROJECTED
ACCRUAL: A
total of 300 patients (150 per arm) will be accrued for this
study.
|
|
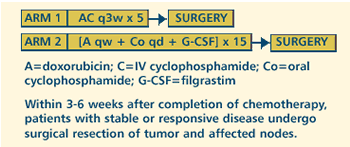
OBJECTIVES
- Compare the response rates in women with inflammatory
or estrogen receptor-negative, locally advanced breast cancer
treated with neoadjuvant doxorubicin and cyclophosphamide
with vs without filgrastim (G-CSF).
- Compare the toxic effects of these regimens.
- Compare the delivered dose intensity of these regimens.
- Evaluate the association between microscopic pathologic
complete response and clinical complete response at the
primary tumor site in these patients.
PARTICIPATION
CRITERIA
- Age and menopausal status not specified
- Histologically confirmed inflammatory or locally advanced
breast cancer
- Stage IIB (T3, N0, M0), IIIA (T3, N1-2, M0 or T0-2, N2,
M0) or IIIB (T4, any N, M0 or any T, N3, M0)
- Unresectable or otherwise appropriate for neoadjuvant
therapy
- Confirmed by core needle or incisional biopsy
- Estrogen receptor-negative if disease is not inflammatory
STUDY
CONTACT
Georgiana
Kehr Ellis, Chair, Ph: 206-288-2048 Southwest Oncology Group
|
| PHASE
III RANDOMIZED STUDY OF NEOADJUVANT FLUOROURACIL, EPIRUBICIN,
AND CYCLOPHOSPHAMIDE VERSUS NEOADJUVANT DOCETAXEL AND EPIRUBICIN
FOLLOWED BY RADIOTHERAPY AND SURGERY IN WOMEN WITH LOCALLY ADVANCED,
INFLAMMATORY, OR LARGE OPERABLE BREAST CANCER Protocol
PROTOCOL
ID: EORTC-10994
PROJECTED
ACCRUAL: A
total of 1,440 patients will be accrued for this study within
3 years.
|
|
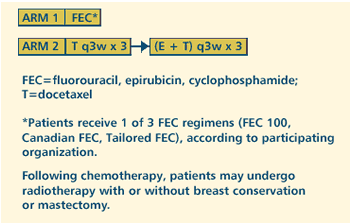
OBJECTIVES
- Compare neoadjuvant fluorouracil, epirubicin and cyclophosphamide
vs docetaxel and epirubicin followed by radiotherapy and
surgery in women with locally advanced, inflammatory or
large operable breast cancer.
- Compare the progression-free survival with these regimens.
- Compare the distant metastasis-free survival and survival
of patients treated with these regimens.
- Compare clinical and pathological responses to these regimens.
- Compare the toxicity of these regimens in these patients.
PARTICIPATION
CRITERIA
- Age 70 and under
- Histologically confirmed breast cancer
- Locally advanced or inflammatory disease (T4a-d, any N,
M0; or any T, N2 or N3, M0)
- Large T2 or T3 breast cancer requiring tumor shrinkage
prior to BCT
STUDY
CONTACTS
Herve
Bonnefoi, Chair, Ph: 011-41-22-382-33-11 EORTC Breast Cancer
Group
Jonas
Bergh, Chair, Ph: 46-8-51776279 Swedish Breast Cancer Group
Barbara
Muster, Chair Ph: 011-41-31-389-9191 Swiss Institute for Applied
Cancer Research
|
| PHASE
III RANDOMIZED STUDY OF NEOADJUVANT FLUOROURACIL/DOXORUBICIN/CYCLOPHOSPHAMIDE
(FAC) VS CYCLOPHOSPHAMIDE/METHOTREXATE/FLUOROURACIL (CMF) FOR
STAGE III BREAST CANCER WITH QUALITY OF LIFE ASSESSED IN PATIENTS
CONVERTED TO CONSERVATIVE SURGERY VS THOSE WHO ARE NOT Protocol
PROTOCOL
IDS: GOCS-08-BR-95-III,
NCI-F95-0036
PROJECTED
ACCRUAL: If
unacceptable toxicity is observed in 10 or more patients,
the study will be closed.
|
|
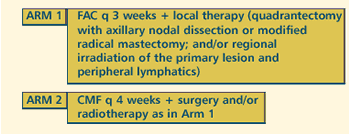
OBJECTIVES
- Compare the response to neoadjuvant therapy with fluorouracil,
doxorubicin and cyclophosphamide (FAC) vs cyclophosphamide,
methotrexate and fluorouracil (CMF) in patients with stage
III breast cancer.
- Compare the rate of breast conservation and local-regional
control with these two regimens.
- Assess the disease-free and overall survival.
- Assess the toxic effects, cosmetic results after conservative
surgery, quality of life and patient compliance.
PARTICIPATION
CRITERIA
- Breast cancer histologically confirmed by tru-cut needle
biopsy
- Measurable, stage III (UICC staging system) disease
- No inflammatory breast cancer
STUDY
CONTACT
Bernardo
A. Leone, Chair, Ph: 54-299-4485247 Grupo Oncologico Cooperativo
del Sur
|
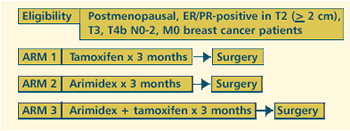
| IMPACT
TRIAL: A RANDOMIZED DOUBLE BLIND TRIAL OF PREOPERATIVE TAMOXIFEN,
ARIMIDEX OR THE COMBINATION IN POSTMENOPAUSAL BREAST CANCER
PATIENTS |
|
STUDY
CONTACT
Ian
Smith, MD, Chair
Royal Marsden Hospital
London, United Kingdom
|
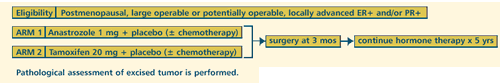
| PROACT:
PREOPERATIVE ARIMIDEX COMPARED TO TAMOXIFEN |
|
STUDY
CONTACT
Aman
Buzdar, MD
MD Anderson Cancer Center
Houston, Texas
|
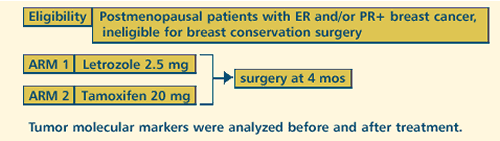
| A
RANDOMIZED DOUBLE-BLIND MULTICENTER STUDY OF PREOPERATIVE TAMOXIFEN
VERSUS FEMARA®(LETROZOLE) FOR POSTMENOPAUSAL WOMEN WITH ER+
AND/OR PGR+ BREAST CANCER INELIGIBLE FOR BREAST-CONSERVING SURGERY.
CORRELATION OF CLINICAL RESPONSE WITH TUMOR GENE EXPRESSION
AND PROLIFERATION |
|
Page
2 of 3
Previous
| Next
Top
Additional
Sections:
1 | 2
| 3 | 4
| 5 | 6
| 7 | 8
| 9 | 10
| 11 | 12
|
|
|

