|
 Home:
Educational
Supplement: Section 5
Home:
Educational
Supplement: Section 5
Section 5
Aromatase
inhibitors in the adjuvant setting
OVERVIEW
Randomized clinical
trials of aromatase inhibitors (AIs) in postmenopausal women with
advanced disease have now demonstrated that, for the first time,
an endocrine intervention has been identified with improved efficacy
compared to tamoxifen. In the next few months, initial results are
expected from the ATAC adjuvant trial comparing anastrozole versus
tamoxifen versus the combination in invasive disease, and several
additional studies are evaluating AIs following adjuvant tamoxifen.
Other trials in noninvasive disease and the high-risk setting are
being planned.
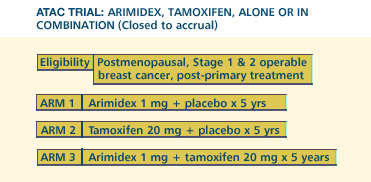
ATAC TRIAL
On the basis
of emerging data in premenopausal women with chemical ovarian ablation
and tamoxifen, one would expect that the combination arm of ATAC
would be superior to the other two arms. On the other hand, there
are some preclinical data suggesting that the combination of tamoxifen
and anastrozole will not be advantageous, so we'll have to wait
for the data. In terms of the single agent ATAC arms, I am impressed
by the data from the large aromatase inhibitor trials in first-line
metastatic breast cancer, and on that basis, we have the hypothesis
that the same type of comparison in the adjuvant setting may have
a similar or magnified outcome.
-Gabriel
Hortobagyi, MD
COMBINATION
ARM OF ATAC
The combination
arm of ATAC is difficult to predict, but the in vivo effects of
Arimidex and tamoxifen are very different - they work through different
mechanisms - and this is the major background basis to believe that
the combination arm might be better.
-J
Michael Dixon, MD, FRCS
ATAC SUBPROTOCOLS
The ATAC
trial has subprotocols looking at bone, lipids and the endometrium
that are appropriately powered to answer those important questions.
However, in the first-line trials of aromatase inhibitors, there
was no increase in bone-related complications such as compression
fractures. I would expect a higher fraction of patients to remain
disease-free in the anastrozole arm, and the main question is the
combined arm - where you may have greater antitumor activity plus
some of the benefits of tamoxifen, for example, on bone. We don't
have many patients in the metastatic setting exposed to aromatase
inhibitors for extended periods of time. If ATAC looks positive,
I would have serious reservations about substituting another aromatase
inhibitor for Arimidex as long-term adjuvant therapy, particularly
because of safety concerns.
-Aman
Buzdar, MD
TOXICITY
PROFILE
Aromatase
inhibitors are better than tamoxifen in the metastatic setting,
not only in terms of antitumor activity but also the safety profile,
because of the risk of thromboembolic complications with tamoxifen
and the agonist effect on the endometrium. Even though there were
few such events in the first-line metastastic trials, the risk of
vaginal bleeding was almost 50 percent lower with anastrozole compared
to tamoxifen.
-Aman
Buzdar, MD
AIs IN WOMEN
WITH THROMBOTIC HISTORIES
From the
data in the metastatic and neoadjuvant setting, it's quite clear
that aromatase inhibitors are superior to tamoxifen. I would expect
that superiority to carry through into the adjuvant setting, although
it may take some time to observe. The use of aromatase inhibitors
in the adjuvant setting is not currently approved, but for high-risk,
ER-positive, postmenopausal patients with a previous stroke or DVT,
we consider substituting an aromatase inhibitor.
-Hyman
Muss, MD
| SUB-PROTOCOLS
OF THE ATAC TRIAL |
|
Pharmacodynamic and pharmacokinetic profiles
Modulation of lipoprotein profiles
Endometrial status
Bone mineral metabolism
Quality of life
|
ATAC
TRIAL PHARMACOKINETIC SUBPROTOCOL DATA
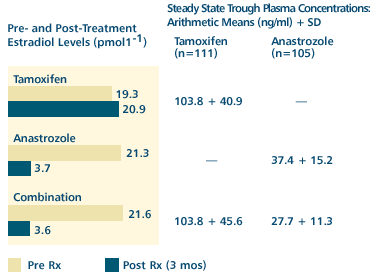
Derived
from Br J Cancer 2001;85(3):317-324. Abstract
The
first main results of the ATAC trial are likely to be presented
at the upcoming San Antonio Breast Cancer Symposium . However,
a just-published subprotocol analysis provides early data
on pharmacologic findings on the combination arm of the
study. Of note is that estradiol suppression is equivalent
in the anastrozole and combination arms, despite a modest
tamoxifen-associated reduction of serum anastrozole levels.
|
SECONDARY
EFFECTS OF AROMATASE INHIBITORS
In ATAC,
I expect some impact of Arimidex on the bones, but probably not
a major effect. Many of our neoadjuvant patients have taken anastrozole
or letrozole for five years, and we have not seen major problems
as far as side effects are concerned. One reason that the UK prevention
study decided not to have a combination arm is that there's not
enough information yet on the safety profile of combining Arimidex
and tamoxifen. Everybody's waiting with baited breath to see the
results of the ATAC study. In the combination arm, there are competing
effects of Arimidex lowering estrogen levels and tamoxifen's estrogen-mimicking
action on bone and lipids. The hope is that any adverse effects
of Arimidex will be countered by the positive effects of tamoxifen.
So, from a theoretical point of view, you'd expect either no effect
or perhaps even a positive effect. In terms of the endometrium,
theoretically the combination should be no worse than tamoxifen
alone. Arimidex causes less vaginal problems than tamoxifen and
no leukorrhea.
-J
Michael Dixon, MD, FRCS
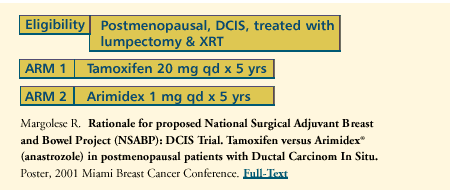
PROPOSED
NSABP DCIS TRIAL: TAMOXIFEN VERSUS ARIMIDEX IN POSTMENOPAUSAL PATIENTS
WITH DUCTAL CARCINOMA IN SITU
PROPOSED
IBIS 2 TRIAL: INTERNATIONAL BREAST INTERVENTION STUDY 2

PROPOSED
NSABP DCIS TRIAL
The driving
force of current research is to move away from the concept that
DCIS is simply a surgical problem - and that if you obtain 10 mm
margins, the patient is cured and no adjuvant therapy is needed.
Even if we take out the index DCIS, the risk for these women to
have another tumor in either breast in the future is at least as
high or higher than the risk for women in the NSABP P-1 prevention
trial. So, chemoprevention in DCIS is an important issue, and we
need to find out how to do this best. The ATAC trial, which has
past accrual and is nearing analysis, will answer the question about
anastrozole in invasive breast cancer. We need to ask the same question
in noninvasive disease.
-Richard
Margolese, MD
PROPOSED
IBIS 2 PREVENTION TRIAL
This will
be an international trial, and we have two good models for big multinational
trials - the AT LAS trial of tamoxifen duration and the ATAC trial
- which ran very similar treatment arms to IBIS 2. The entry criteria
of IBIS 2 will be similar to IBIS 1, but restricted to postmenopausal
women. We don't use the Gail model. Family history is factored in,
along with nulliparity and pathologic entities such as atypical
hyperplasia and LCIS. We're also adding a new category of women
who have mammographic dysplasia covering 50 percent or more of their
breasts. That links it in nicely with screening which is really
where prevention belongs. Screening programs should not only detect
small cancers, but also identify women at high risk and offer them
preventative strategies. IBIS 2 is also going to have a DCIS component.
The trial will enter 12,000 high-risk women and another 4,000 patients
with DCIS.
-Jack
Cuzick, PhD
Page
1of 2
Next
page
Top
Additional
Sections:
1 | 2
| 3 | 4
| 5 | 6
| 7 | 8
| 9 | 10
| 11 | 12
|
|

