|
 Home:
Educational
Supplement: Section 11
Home:
Educational
Supplement: Section 11
Section 11
Other
Breast Cancer Clinical Trials
| PHASE
IB RANDOMIZED, MULTIPLE-DOSE PHARMACODYNAMIC (BIOMARKER MODULATION)
STUDY OF LY353381 HYDROCHLORIDE, TAMOXIFEN, AND PLACEBO IN POSTMENOPAUSAL
WOMEN WITH NEWLY DIAGNOSED BREAST CANCER Protocol
PROTOCOL
IDS: KUMC-7813-99,
NCI-P00-0158, KUMC-HSC-7419-98
PROJECTED
ACCRUAL: A
minimum of 120 patients (60 per treatment phase) will be accrued
for this study within 18 months.
|
|

OBJECTIVES
- Determine whether LY353381 hydrochloride or tamoxifen
administered in the interval between biopsy and re-excision
alters the expression of tissue biomarkers relative to placebo
controls in postmenopausal women with newly diagnosed breast
cancer.
PARTICIPATION
CRITERIA
- Histologically confirmed noninvasive or small invasive
breast cancer
- Low or intermediate grade (ductal carcinoma in situ,
T1 or T2) OR
- Estrogen and/or progesterone receptor-positive
- Largest mass no greater than 5 cm
- Clustered microcalcifications as only abnormality allowed
with no upper size limit
- If no distinction between mass and microcalcifications,
size as 1 lesion
- No evidence of metastases from any malignancy
- 18 and older
- Postmenopausal
- At least 1 year since chemotherapy, prior aromatase inhibitors,
antiestrogens or LH agonists/antagonists
- No concurrent hormone replacement therapy or oral contraceptives
- Lumpectomy or mastectomy must be planned for 2-6 weeks
from start of study
STUDY
CONTACT
Carol
J. Fabian, Chair Ph: 913-588-7791
University of Kansas Medical Center
|
| RANDOMIZED
STUDY OF METHODS IN EDUCATION FOR BREAST CANCER GENETICS Protocol
PROTOCOL
IDS: NCI-99-C-0081,
MB-NAVY-B99-015, NCI-NMOB-9811
PROJECTED
ACCRUAL: A
total of 120 participants (60 per arm) will be accrued for
this study within 18-24 months.
|
OBJECTIVES
- Compare the effect of two different methods of providing
education to persons enrolling in a breast cancer genetics
program.
PARTICIPATION
CRITERIA
Diagnosis
of breast cancer or ductal carcinoma in situ at age 45 or
under OR Diagnosis of ovarian cancer at age 50 or under OR
Diagnosis of breast cancer with bilateral disease or multiple
primaries or breast cancer and ovarian cancer in the same
individual OR Diagnosis of breast or ovarian cancer AND At
least one first- or second-degree relative with breast cancer
diagnosed at age 45 or under or ovarian cancer at age 50 or
under OR Three relatives in the same lineage with breast or
ovarian cancer where each affected individual is a first-
or second-degree relative to another of the affected individuals
OR First- or second-degree male relative with breast cancer
diagnosed at any age OR Women of Ashkenazi Jewish descent
who meet any of the above criteria with specified ages of
onset of 50 for breast cancer and any age for ovarian cancer
OR Male with breast cancer diagnosed at any age OR Documented
BRCA mutation in the family.
PROTOCOL
This is
a randomized study. Participants are randomized to one of
two different education methods. All participants complete
a pretest questionnaire, then attend a breast cancer genetics
education and counseling session. A post test questionnaire
is also completed. Participants may then choose to undergo
germline BRCA testing. Participants are followed at 1 week
and 3, 6, and 12 months after receiving results of BRCA germline
testing.
STUDY
CONTACT
Pamela
Klein, Ph: 301-295-3899
Division of Clinical Sciences, Medicine Branch
Bethesda, Maryland
|
| PHASE
II PILOT STUDY OF MRI-GUIDED FOCUSED ULTRASOUND ABLATION IN
WOMEN WITH STAGE I-IIIA BREAST CANCER Protocol
PROTOCOL
IDS: TXS-G990184,
NCI-V00-1643, DFCI-99029, MDA-ID-99137, TXS-1999-P-009925/10
PROJECTED
ACCRUAL: A
total of 15 patients.
|
OBJECTIVES
- Determine the incidence and severity of adverse events
during and after MRI-guided focused ultrasound ablation
in women with stage I-IIIA breast cancer.
- Determine the ability to accurately and thoroughly coagulate
a target volume of breast carcinoma, in terms of real-time
target volume temperature profile, follow-up MRI, and histology,
using this procedure.
- Compare the appearance of gross and microscopic histopathologic
tissue post coagulation with the pre- and post coagulation
magnetic resonance appearance of the targeted volume and
measure any residual cancer cells in patients following
this procedure.
- Determine patient acceptance of this procedure in terms
of positioning, pain, safety, and follow-up cosmesis.
PARTICIPATION
CRITERIA
- Histologically confirmed invasive breast cancer (T1, N0-2,
M0)
- Single focal lesion no greater than 3.5 cm in diameter
by MRI
- No lesions difficult to target, defined as less than 1
cm from skin, nipple, or rib cage
- No microcalcifications as sole sign of disease
- No extensive intraductal components on core biopsy
- No breast implants
- At least 3 months since prior chemotherapy
- Concurrent hormone replacement therapy allowed, concurrent
tamoxifen allowed
- No concurrent steroids
- No prior external radiotherapy or laser therapy to ipsilateral
breast
PROTOCOL
Patients
undergo MRI-guided focused ultrasound (MRgFUS) ablation of
the breast lesion using a series of pulses. Within 72 hours
after MRgFUS procedure, patients undergo gadolinium-enhanced
MRI to evaluate ablation borders. Within 7-10 days after MRgFUS
procedure, patients undergo an ultrasound exam, and guide
wires may be placed to assist in pre-surgical lesion localization.
Within 10-21 days after MRgFUS procedure, patients undergo
segmental resection or mastectomy. Patients are followed at
5-10 days post-surgery.
STUDY
CONTACT
Pamela
Klein, Ph: 301-295-3899
Division of Clinical Sciences, Medicine Branch
Bethesda, Maryland
|
| PHASE
III RANDOMIZED STUDY OF HORMONE REPLACEMENT THERAPY IN MENOPAUSAL
OR PERIMENOPAUSAL WOMEN WITH PRIOR STAGE O-II BREAST CANCER
Protocol
PROTOCOL
IDS: ROC-HABITS,
EU-98077
PROJECTED
ACCRUAL: A
total of 1,300 patients will be accrued for this study over
5-6 years.
|
|
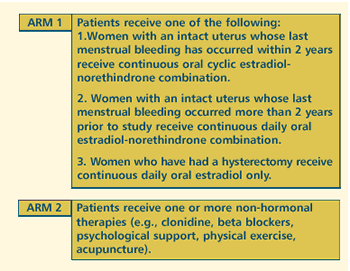
OBJECTIVES
- Evaluate the safety of hormone replacement therapy in
terms of risk of recurrence in women with previously treated,
nonrecurrent stage O-II breast cancer.
- Compare this regimen with non-hormonal symptomatic treatment
in terms of quality of life and risk of death in this patient
population.
PARTICIPATION
CRITERIA
- Menopausal or perimenopausal
- Hormone receptor status: positive, negative, or unknown
- History of stage O-II breast cancer with no more than
four involved axillary nodes if nodal status and number
of nodes investigated is known
- Currently without evidence of disease
- No concurrent chemotherapy
- Prior hormone replacement therapy (HRT) allowed if stopped
no more than 4 weeks after breast cancer diagnosis and at
least 3 months prior to study
- No prior HRT begun after breast cancer diagnosis
- No concurrent hormonal therapy for breast cancer except
tamoxifen or Toremifene
- No concurrent radiotherapy
STUDY
CONTACT
C. Rageth,
Ph: 0041 1 733 21 76
International Breast Cancer Study Group,
Spital Limmattal, Switzerland
See PDQ for other investigators
|
| PHASE
III STUDY OF THE EFFECT OF MENSTRUAL CYCLE TIMING WITH BREAST
SURGERY ON PROGNOSIS IN PREMENOPAUSAL WOMEN WITH STAGE I, II,
OR III BREAST CANCER Protocol
PROTOCOL
IDS: UCLA-9810046,
NCI-G00-1724, UCSD-985772
PROJECTED
ACCRUAL: Approximately
400 patients will be accrued for this study over 2.5 years.
|
OBJECTIVES
- Determine if the timing of breast surgery during the menstrual
cycle impacts disease recurrence, progression, or death
among different racial groups in premenopausal women with
stage I, II, or III breast cancer.
- Determine if definitive breast cancer surgeries (e.g.,
lumpectomy or mastectomy) performed during the follicular
phase result in poorer prognosis (recurrence, disease progression,
or death) compared with surgeries performed during the midcycle
or luteal phases in this patient population.
PARTICIPATION
CRITERIA
- Premenopausal
- Hormone receptor status: Not specified
- Histologically confirmed stage I, II, or III primary breast
cancer undergoing breast surgery
- Invasive disease (e.g., lobular or ductal), no bilateral
disease no distant metastases
- Regular menses (no amenorrhea of greater than 90 days)
without hormone replacement
- Documented last menstrual period
- No preoperative chemotherapy
- No concurrent hormonal replacement therapy
- No concurrent interruptive oral contraceptive use of less
than 3 months
PROTOCOL
This is
a multicenter study. Patients undergo either fine needle aspiration
concurrently with definitive breast surgery (mastectomy or
lumpectomy) or needle directed excisional biopsy followed
by definitive breast surgery. Patients undergo serum collection
for hormonal analysis preoperatively, 24 hours post operatively,
at days 7 and 14, and at 3 months and urine collection for
hormonal analysis beginning 24 hours prior to surgery and
continuing daily until the onset of the next menses.
Patients
complete a 30 minute telephone interview regarding medical,
family, occupational, and reproductive history, and lifestyle
habits (e.g., diet, exercise, environmental exposures). Beginning
24 hours prior to surgery and continuing until the onset of
the next menses, patients complete a menstrual cycle journal
indicating the start and length of menses.
Patients
undergoing mastectomy are followed every 3 months for 1 year,
every 6 months for 1 year, and then annually thereafter. Patients
undergoing adjuvant therapy are followed every 3 months for
3 years, and then every 6 months thereafter or every 4 months
for 2 years and then every 6 months thereafter.
STUDY
CONTACT
Helena
R. Chang, Ph: 310-794-5624
Jonsson Comprehensive Cancer Center, UCLA
Los Angeles, California
|
| RANDOMIZED
STUDY OF STRESS REDUCTION IN OLDER WOMEN WITH STAGE II, III,
OR IV BREAST CANCER Protocol
PROTOCOL
IDS: SJHCH-TM1,
NCI-V00-1618
PROJECTED
ACCRUAL: Approximately
166 patients (83 per treatment arm) will be accrued for this
study over 1.5 year.
|
|

OBJECTIVES
- Compare the effects of active stress reduction with transcendental
meditation vs basic breast cancer education on quality of
life and survival time in older women with stage II, III,
or IV breast cancer.
- Determine behavioral mechanisms that may mediate the effects
of stress reduction on survival in these patients.
- Determine baseline variables that contribute to predicting
survival time in these patients.
PARTICIPATION
CRITERIA
- Age: 55 and over
- Hormone receptor status: Not specified
- Diagnosis of stage II, III, or IV breast cancer
- No brain or CNS metastases
STUDY
CONTACT
Rhoda
Pomerantz, Ph: 773-665-3606
St. Joseph Health Centers & Hospital
Chicago, Illinois
|
| CORRELATION
OF MENSTRUAL CYCLE PHASE AT THE TIME OF SURGERY WITH DISEASE-FREE
SURVIVAL IN WOMEN WITH STAGE I/II BREAST CANCER Protocol
PROTOCOL
IDS: NCCTG-N9431
PROJECTED
ACCRUAL: A
total of 1,100 patients will be accrued for this study. Approximately
90 patients are expected to undergo two stages of surgery
that do not occur within the same phase of the menstrual cycle.
|
OBJECTIVES
- Document menstrual phase (follicular vs luteal) by circulating
hormones and menstrual history in premenopausal women at
the time of primary surgery for stage I/II breast cancer.
- Correlate menstrual phase at primary surgery with 5-year
disease-free survival in these patients.
- Compare the menstrual cycle data obtained by hormone levels
and study-specific menstrual cycle history with information
recorded in the general written record.
- Compare the menstrual cycle data for these women (i.e.,
hormone levels and cycle history) with the data for the
general population.
- Estimate the disease-free survival of women who undergo
a 2-stage surgical procedure with cancer found at both stages
when the surgery is not confined to the same menstrual cycle
phase.
PARTICIPATION
CRITERIA
- Age: 18 to 55
- Premenopausal
- Hormone receptor status: Not specified
- Pathologically confirmed stage I/II breast cancer
- No prior neoadjuvant therapy
- Concurrent chemotherapy allowed At least 3 months since
oral contraceptives
- Concurrent radiotherapy allowed
- Complete surgical resection required prior to entry
- One or two stage procedure (i.e., open biopsy followed
immediately or later by mastectomy or breast-conserving
approach)
- Two-step registration required for patients undergoing
two-stage procedure
- Fine needle aspiration (FNA), stereotactic, or core
needle biopsy is allowed at any time prior to open biopsy
- Sentinel node dissection/axillary node dissection
allowed.
PROTOCOL
This is
a multicenter study. Hormone levels and menstrual history
are obtained within one calendar day to surgery. Patients
undergo either one-stage surgery (open biopsy followed by
mastectomy or breast conserving surgery) or two-stage surgery.
Patients
complete a questionnaire 6 months after surgery to assess
the extent of adjuvant therapy received (if any), and a questionnaire
12 months after surgery to assess recurrence of breast cancer
and vital status. Patients are followed annually thereafter
for 10 years.
STUDY
CONTACT
Clive
S Grant, Chair, Ph: 507-284-2644
North Central Cancer Treatment Group
D Lawrence
Wickerham, Chair, Ph: 412-330-4600
National Surgical Adjuvant Breast and Bowel Project
|
| DIAGNOSTIC
RANDOMIZED STUDY OF RADIOACTIVE SEED LOCALIZED BREAST BIOPSY
VERSUS NEEDLE LOCALIZED BREAST BIOPSY IN PATIENTS WITH NONPALPABLE
BREAST LESIONS Protocol
PROTOCOL
IDS: MCC-12114,
NCI-G00-1808, MCC-IRB-549 q
PROJECTED
ACCRUAL: A
total of 100 patients (50 per arm).
|
|
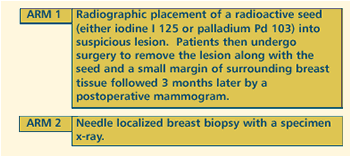
OBJECTIVES
- Compare the effect of low dose radioactive seed localized
breast biopsy versus needle localized breast biopsy on operative
time and tissue loss in patients with nonpalpable breast
lesions.
- Compare the cost-effectiveness of these diagnostic methods
in these patients.
- Demonstrate that radioactive seed localization allows
for elimination of specimen X-ray in these patients.
- Demonstrate that radioactive seeds may be placed safely
for 1-7 days prior to surgical removal in these patients.
PARTICIPATION
CRITERIA
- Suspicious nonpalpable breast lesion requiring breast
biopsy for diagnosis OR nonpalpable breast lesion that is
not amenable to core biopsy or advanced breast biopsy instrumentation
(ABBI) excision.
STUDY
CONTACT
Charles
E. Cox, Ph: 813-972-8480
H Lee Moffitt Cancer Center and Research Institute
Tampa, Florida
|
| DIAGNOSTIC
STUDY OF MAGNETIC RESONANCE IMAGING IN WOMEN WITH SUSPECTED
BREAST TUMORS Protocol
PROTOCOL
IDS: UPCC-ACR-6883
PROJECTED
ACCRUAL: A
total of 1500 patients will be accrued for this study over
4.25 years.
|
OBJECTIVES
- Evaluate the performance of breast magnetic resonance
imaging (MRI) in conjunction with mammography for the detection
and characterization of lesions in women with suspicious
mammographic or clinical examinations.
- Assess the incremental value of breast MRI to determine
the local extent of cancer in these patients.
- Assess the value of breast MRI to determine the prevalence
and characteristics of incidental enhancing lesions in the
remainder of the breast.
PARTICIPATION
CRITERIA
- Suspicious mammographic finding or palpable abnormality
OR Suspicious clinical or ultrasound finding without associated
benign mammographic features
- May have more than one suspicious lesion based on mammography
or clinical exam if an index lesion is present
- Mammogram within 2 months prior to MRI scan and copy of
films required of all patients 30 years of age and over
- Eligibility maintained if patient meets above criteria
and has had: Breast implant, prior benign excisional or
core biopsy at least 6 months prior to study, fine needle
aspiration performed at any time, cancer in the contralateral
breast, no history of prior breast cancer in the study breast
PROTOCOL
This is
a multicenter study.
Patients
undergo a high resolution 3D post contrast magnetic resonance
imaging (MRI) scan. Patients with enhancing abnormalities
undergo a dynamic scan no less than 18 hours later. Some patients
may require a third scan if a core biopsy is to be performed.
Patients
who are ultimately found to have cancer are assessed for extent
of cancer including measurement of the index lesion and identification
of other present foci of cancer in relation to the index lesion.
Further histological diagnosis of index lesions is determined
by MRI-guided needle localization excisional biopsy. Patients
with benign needle biopsy are followed for 2 years. Patients
with benign primary lesions receive a follow up MRI scan 1
year after the initial scan. Patients with benign primary
lesions and incidental enhancing lesions (IEL) are followed
at 2 years. Patients with negative needle biopsies not yielding
a specific diagnosis and who do not undergo subsequent excisional
biopsy are followed yearly for 2 years.
STUDY
CONTACT
Mitchell
Schnall, Ph: 215-662-7238
University of Pennsylvania Cancer Center
Philadelphia, Pennsylvania
|
| PHASE
II STUDY OF ORAL VINORELBINE IN ELDERLY WOMEN WITH STAGE IV
BREAST CANCER Protocol
PROTOCOL
IDS: NCCTG-N003A
PROJECTED
ACCRUAL: A
total of 12-35 patients will be accrued for this study within
7-18 months.
|
OBJECTIVES
- Determine the objective response rate in elderly women
with stage IV breast cancer treated with oral vinorelbine.
- Determine the toxicity profile of this drug in these patients.
- Determine the time to progression in these patients treated
with this drug.
- Assess these patients' pre f e rences with re g a rd to
this oral drug .
- Determine the quality of life of these patients treated
with this drug.
- Assess individual variation in responses (toxicity and/or
activity), pharmacokinetic parameters, and/or biologic correlates
due to genetic differences in enzymes involved in the transport,
metabolism, and/or mechanism of action of this drug in these
patients.
PARTICIPATION
CRITERIA
- Age 65 and over
- Histologically or cytologically confirmed stage IV breast
cancer
- Eligible to receive first- or second-line chemotherapy
- Measurable disease: At least 1 lesion that can be accurately
measured in at least 1 dimension as at least 20 mm in longest
diameter. Must be completely outside prior irradiation port
unless there is proof of progressive disease after completion
of prior radiotherapy
PROTOCOL
Patients
receive oral vinorelbine weekly. Courses repeat every 4 weeks
in the absence of disease progression or unacceptable toxicity.
STUDY
CONTACT
Michael
J. O'Connell, Chair Ph: 507-284-2511
North Central Cancer Treatment Group
|
| PILOT
SCREENING STUDY OF BREAST IMAGING OUTCOME MEASURES IN WOMEN
AT HIGH GENETIC RISK OF BREAST CANCER Protocol
PROTOCOL
IDS: NCI-01-C-0009
PROJECTED
ACCRUAL: Approximately
200 participants (100 BRCA1/2 mutation carriers and 100 BRCA1/2
mutation non-carriers).
|
OBJECTIVES
- Determine whether breast imaging outcome measures in women
who are BRCA1 or BRCA2 mutation carriers and non-carriers
can be used to define a high-risk imaging phenotype.
- Assess the use of positron emission tomography imaging
of breast lesions detected by mammography or magnetic resonance
imaging, normal contralateral breast tissue, and normal
ovarian tissue in BRCA1/2 carriers and non-carriers.
- Assess the use of breast duct lavage to obtain epithelial
cell samples for cytologic evaluation and molecular/genetic
studies in high-risk premenopausal women.
- Compare imaging findings with histologic or cytologic
findings from these participants.
PARTICIPATION
CRITERIA
- Age Range: 18 to 49
- Previous participation on protocol NCI-78-C-0039 or NCI-99-C-0081
- Known carrier or at least a 50% probability of carrying
a BRCA1 or BRCA2 mutation
- Must agree to release results of genetic counseling for
stratification purposes
- Have undergone genetic counseling and risk assessment
- At least 6 months since prior steroids, selective estrogen
receptor modulators, or hormonal agents, including the following:
tamoxifen, raloxifene, estrogen, DHEA, anabolic steroids,
oral contraceptives, Depoprovera, progestin IUD, oral progestin,
Norplant, or drugs to induce ovulation
PROTOCOL
Participants
undergo a physical exam including exam of breast and pelvis,
standard four-view mammogram, breast magnetic resonance imaging
(MRI), CA-125 level determination and transvaginal color doppler
ultrasonography.
Participants
with abnormal mammogram and/or MRI results are asked to undergo
positron emission tomography scans of the breast and asymptomatic
ovaries. Breast duct lavage fluid is collected from all participants
for cytologic analysis. Participants undergo repeat screening
studies annually for 3 years.
STUDY
CONTACT
Ruthann
M. Giusti, Chair Ph: 301-496-1611
Division of Cancer Epidemiology and Genetics
|
| RANDOMIZED
STUDY OF GABAPENTIN FOR THE CONTROL OF HOT FLASHES AND OTHER
VASOMOTOR SYMPTOMS IN WOMEN WITH BREAST CANCER Protocol
PROTOCOL
IDS: URCC-U2101,
NCI-P01-0183
PROJECTED
ACCRUAL: A
total of 408 patients (136 per arm) will be accrued for this
study within 18 months.
|
|

OBJECTIVES
- Compare the effectiveness and side effects of 2 different
doses of gabapentin vs placebo for the control of hot flashes
and other vasomotor symptoms in women with breast cancer.
- Compare quality of life, anxiety, and depression in patients
treated with these regimens.
PARTICIPATION
CRITERIA
- Diagnosis of breast cancer
- Experiencing 2 or more hot flashes per day for at least
1 week
- No concurrent clonidine or venlafaxine
STUDY
CONTACT
Kishan
J. Pandya, Chair Ph: 716-275-9319
University of Rochester Cancer Center
|
| PHASE
III RANDOMIZED STUDY OF BEVACIZUMAB WITH CAPECITABINE VERSUS
CAPECITABINE ALONE IN WOMEN WITH PREVIOUSLY TREATED METASTATIC
BREAST CANCER Protocol
PROTOCOL
IDS: GENENTECH-AVF2119g,
GUMC-00299, MSKCC-01008, UAB-0028, UAB-F001009003
PROJECTED
ACCRUAL: A
total of 400 patients will be accrued for this study within
12 months.
|
OBJECTIVES
- Compare the efficacy of bevacizumab with capecitabine
vs capecitabine alone in women with previously treated metastatic
breast cancer, as measured by time to disease progression,
objective response rate, duration of response, 1-year survival,
and duration of survival.
- Compare the safety of these regimens in these patients.
- Compare the pharmacokinetics of these regimens in a subset
of these patients.
- Determine the pharmacodynamics of bevacizumab with capecitabine
in a subset of these patients.
- Compare the extent of change in quality of life of patients
treated with these regimens.
PARTICIPATION
CRITERIA
- Age: 18 and over
- Histologically confirmed progressive metastatic breast
cancer
- Previously treated with 1-2 conventional chemotherapy
regimens for metastatic disease
- Previously treated with both an anthracycline (or anthracenedione)
and taxane OR
- No prior chemotherapy for metastatic disease if previously
treated with an adjuvant anthracycline (or anthracenedione)
and taxane regimen and if relapse occurred within 12 months
of completing adjuvant therapy
- Bidimensionally measurable disease
- No HER2-positive disease (3+ by immunohistochemistry or
positive by FISH) unless previously relapsed after trastuzumab
(Herceptin)
PROTOCOL
Arm I:
Oral capecitabine twice daily on days 1-14.
Arm II:
Chemotherapy as in arm I plus bevacizumab IV over30-90 minutes
on day 1.
Treatment
repeats in both arms every 21 days for up to 35 courses in
the absence of disease progression or unacceptable toxicity.
Patients with progressive disease in arm II may continue to
receive bevacizumab alone or in combination with a new chemotherapy
regimen or other treatment.
STUDY
CONTACT
Ginny Langmuir, Chair Ph: 650-225-4985
Genentech Inc.
|
| PHASE
III RANDOMIZED STUDY OF EPOETIN ALFA IN ANEMIC PATIENTS WITH
ADVANCED CANCER UNDERGOINGCHEMOTHERAPY Protocol
PROTOCOL
IDS: NCCTG-979253,
NCI-P98-0133
PROJECTED
ACCRUAL: There
will be 300 patients (150 patients per arm) accrued into this
study over 11 months.
|
OBJECTIVES
- Determine whether epoetin alfa treatment improves the
quality of life in anemic patients who are undergoing chemotherapy
for advanced malignancy.
- Determine whether epoetin alfa increases hemoglobin levels
and decreases transfusion requirements in these patients.
- Validate or refute the use of an algorithm using pre-
and post-treatment epoetin alfa, ferritin, and hemoglobin
levels to predict 16 weeks response or no response to therapeutic
doses of epoetin alfa as set forth by these patients.
- Explore whether anemic patients receiving platinum-containing
chemotherapy regimens experience less nephrotoxicity if
they receive concurrent epoetin alfa compared to those who
receive placebo.
PARTICIPATION
CRITERIA
- Age: 18 and over
- Anemic: Hemoglobin in males less than 11.5 g/dL; hemoglobin
in females less than 10.0 g/dL
- Histologically confirmed advanced malignancy
- Currently receiving myelosuppressive, cytotoxic chemotherapy
for advanced cancer
PROTOCOL
Patients
receiving chemotherapy are randomized to receive epoetin alfa
subcutaneously once a week for a maximum of 16 weeks (Arm
I) or placebo subcutaneously once a week for a maximum of
16 weeks (Arm II).
STUDY
CONTACT
Thomas
E. Witzig, Chair Ph: 507-284-2176
North Central Cancer Treatment Group
|
| PHASE
I STUDY OF DOCETAXEL IN PATIENTS WITH ADVANCED SOLID TUMORS
AND VARYING DEGREES OF ABNORMAL LIVER FUNCTION Protocol
PROTOCOL
IDS: CHNMC-PHI-08,
NCI-T96-0028H, LAC-USC-PHI-08
PROJECTED
ACCRUAL: A
maximum of 45 patients will be accrued for this study within
12-18 months.
|
OBJECTIVES
- Determine the maximum tolerated dose of docetaxel in patients
with advanced solid tumors and varying degrees of liver
dysfunction.
- Determine the effects of liver dysfunction in these patients
on the plasma pharmacokinetics and pharmacodynamics of this
therapy.
- Determine the utility of indocyanine green clearance and
lidocaine metabolism as indicators of hepatic elimination
of docetaxel in these patients.
PARTICIPATION
CRITERIA
- Age 18 and over
- Solid tumor that is refractory to standard therapy or
for which no standard therapy exists
- Eligible tumors, include, but are not limited to, the
following:
- Breast
- Ovarian
- Head and neck
- Non-small cell lung cancer
- Abnormal liver function
- Control patients with normal liver function are enrolled
- Brain metastases allowed if controlled by radiotherapy
or surgery and neurologic status currently stable
- No prior bone marrow transplantation
- At least 4 weeks since prior chemotherapy (6 weeks for
mitomycin or nitrosoureas) and recovered
- At least 4 weeks since prior radiotherapy and recovered
(no prior radiotherapy to more than 25% of bone marrow)
PROTOCOL
This is
a dose-escalation, multicenter study. Patients are stratified
according to liver function (normal vs mild vs moderate vs
severe).
Patients
receive docetaxel IV over 1 hour. Courses repeat every 3 weeks
in the absence of disease progression or unacceptable toxicity.
Patients who achieve complete remission (CR) receive 2 additional
courses past CR.
Within
each abnormal liver function stratum, cohorts of 3-6 patients
receive escalating doses of docetaxel until the maximum tolerated
dose is determined.
The MTD
is defined as the dose preceding that at which 2 of 6 patients
experience dose-limiting toxicity. Within each abnormal liver
function stratum, more than 6 patients are treated at the
MTD, if possible. Patients in the normal liver function stratum
are included as control patients and are followed for toxicity,
but do not undergo dose escalation.
STUDY
CONTACT
James
H. Doroshow, Chair Ph: 626-359-8111
Beckman Research Institute, City of Hope
Los Angeles, California, U.S.A.
|
Additional
clinical trials
Top
Additional
Sections:
1 | 2
| 3 | 4
| 5 | 6
| 7 | 8
| 9 | 10
| 11 | 12
|
|
|

