|
 Home:
Educational
Supplement: Section 3
Home:
Educational
Supplement: Section 3
Section 3
Radiation
therapy for primary breast cancer
OVERVIEW
A number of
important research questions on the role of radiation therapy in
primary breast cancer are being evaluated in current clinical trials.
One of the few local therapy questions addressed by the 2000 NIH
Consensus Conference was postmastectomy radiation therapy in women
with one to three positive nodes. A major Intergroup trial now addressing
this question is particularly salient because of several recently
reported randomized studies suggesting a survival benefit in this
population.
The research
question of whether improved local disease control affects long-term
survival is provocative, but consistent with data from the International
Breast Cancer Overview that demonstrated fewer breast cancer deaths
in women receiving local radiation therapy. This effect was confounded
by an increased mortality from cardiovascular complications, which
was attributed to prior, imprecise radiation therapy techniques.
Many researchers believe that the benefits of radiation therapy
- like adjuvant systemic therapy - exist in a continuum, with the
greatest absolute benefit occurring in patients with the greatest
risk for recurrence.
RADIATION
THERAPY OVERVIEW
When you obtain
better local control with radiotherapy or with surgery that reduces
the risk of local recurrence, you do get some decrease in the long-term
mortality from breast cancer. An absolute reduction of about 20
percent in local recurrence seems to go with an absolute reduction
of around about five percent in long-term mortality from breast
cancer 10 or 15 years later.
So, although
the meta-analysis of the older radiotherapy trials suggested no
net benefit, when you look separately at the breast deaths and the
nonbreast deaths, it actually shows that better local control does
matter in terms of long-term breast cancer survival.
-Richard
Peto, FRS
POSTMASTECTOMY
RADIATION THERAPY
Recent trials
have shown a survival benefit following radiotherapy in all node-positive
women, but the degree of benefit is unclear in patients with one
to three positive nodes. Part of the dilemma is based upon the discrepancy
in the rates of locoregional failure without radiotherapy in those
trials in comparison to failure rates reported in American series.
The recent report by Recht and colleagues of the patterns of failure
found in studies conducted by the Eastern Cooperative Oncology Group
notes that the risk of locoregional failure was 13 percent at 10
years in patients with one to three positive nodes. Although this
is comparable to the 16 percent actuarial rate seen in the British
Columbia trial at 10 years, it is strikingly different from the
Danish studies, where the crude rates of locoregional recurrence
were approximately 30 percent.
Based upon these
results, the statement produced from the consensus conference convened
by the American Society for Therapeutic Radiology and Oncology to
address the controversies regarding patient selection for postmastectomy
radiotherapy stated that while there was a consensus that patients
with four or more positive lymph nodes should receive radiation
therapy, the data were less clear for patients with one to three
positive nodes.
-Lori
Pierce, MD
2000 NIH Consensus Conference. Abstract
CONTINUUM
OF RADIOTHERAPY BENEFITS
Some of the
controversy in the one to three node subset is quite akin to what
happened in adjuvant chemotherapy early on in its development. We
used to think that patients with large numbers of nodes benefited,
but patients with small numbers of nodes didn't. Then we recognized
that patients with small numbers of nodes benefited, but we thought
patients with no nodes didn't. Now we recognize that even patients
with no nodes in certain subsets can benefit. This principle applies
across the spectrum of disease that systemic chemotherapy reduces
risk of failure; however, the absolute benefit is harder and harder
to see as the absolute risk of failure gets smaller. I think the
same must be true in postmastectomy chest wall radiation. It almost
certainly has to help patients with one to three positive nodes.
The question is, "What is their absolute risk of failure and
is the benefit of the reduction conferred by postmastectomy chest
wall radiation worth taking?" With the uncertainty surrounding
the one to three node-positive group, we have proposed a trial for
both pre- and postmenopausal patients to assess this. This trial
- sponsored through SWOG - is now open and will be run through the
Intergroup. There is a huge subgroup of women out there who fit
into this category, and we hope that clinicians will enroll their
patients in this study.
-Allen
Lichter, MD
Presentation at 2000 Lynn Sage Breast Cancer Symposium
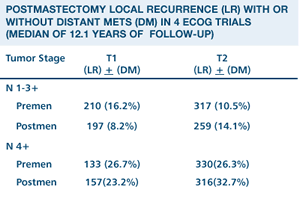
Recht
A et al. Locoregional failure 10 years after mastectomy and adjuvant
chemotherapy with or without tamoxifen without irradiation: Experience
of the Eastern Cooperative Oncology Group. J Clin Oncol
1999;17:1689-1700. Abstract
| DANISH
BREAST CANCER COOPERATIVE GROUP DBCG 82B RANDOMISED TRIAL: POSTOPERATIVE
RADIOTHERAPY IN HIGH-RISK PREMENOPAUSAL BREAST CANCER PATIENTS
GIVEN ADJUVANT CHEMOTHERAPY |

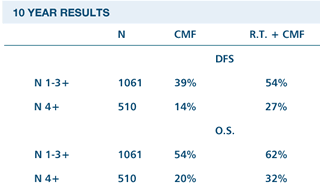
Overgaard
M et al. Postoperative radiotherapy in high-risk premenopausal
women with breast cancer who receive adjuvant chemotherapy.
N Engl J Med 1997;337:949-955. Abstract
|
| DANISH
BREAST CANCER COOPERATIVE GROUP RANDOMISED DBCG 82C TRIAL: POSTOPERATIVE
RADIOTHERAPY IN HIGH-RISK POSTMENOPAUSAL BREAST CANCER PATIENTS
GIVEN ADJUVANT TAMOXIFEN |

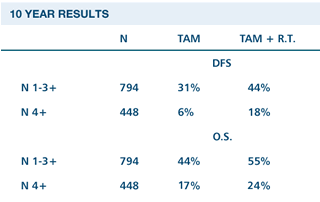
Overgaard
M et al. Postoperative radiotherapy in high-risk post-menopausal
breast cancer patients given adjuvant tamoxifen: Danish
Breast Cancer Cooperative Group DBCG 82c randomised trial.
Lancet 1999;353:1641-1648. Abstract
|
| BRITISH
COLUMBIA RANDOMIZED TRIAL: ADJUVANT RADIOTHERAPY AND CHEMOTHERAPY
IN NODE-POSITIVE PREMENOPAUSAL WOMEN WITH BREAST CANCER |

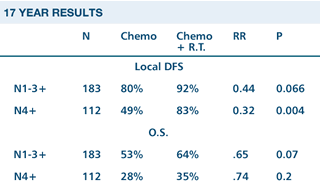
Ragaz
J et al. Adjuvant radiotherapy and chemotherapy in node-positive
premenopausal women with breast cancer. N Engl J
Me 1997; 337:956-962 .Abstract
Ragaz J et al. Postmastectomy radiation (RT) outcome
in node (N) positive breast cancer patients among N 1-3
versus N4+ subset: Impact of extracapsular spread (ES).
Update of the British Columbia randomized trial. Proc
ASCO 1999; 274. Abstract
|
| PHASE
III RANDOMIZED STUDY OF POSTMASTECTOMY RADIOTHERAPY IN WOMEN
WITH STAGE II BREAST CANCER WITH ONE TO THREE POSITIVE NODES
Protocol
PROTOCOL
IDS: SWOG-S9927, GUMC-00223
PROJECTED
ACCRUAL: A total of 2,500 patients (1,250 per treatment
arm) will be accrued for this study over 5 years.
|
|
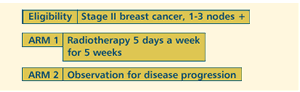
OBJECTIVES
- Compare overall and disease-free survival in women with
stage II breast cancer with one to three positive nodes
with or without radiotherapy following mastectomy and adjuvant
chemotherapy.
- Compare local-regional control in these patients with
these treatment regimens.
- Assess the potential toxicities of radiotherapy in this
patient population.
PARTICIPATION
CRITERIA
- Histologically confirmed stage II adenocarcinoma of the
breast (T1-2, N1, MO)
- Primary tumor no greater than 5 cm
- At least 1 but no more than 3 positive axillary lymph
nodes
- Nodes cannot be positive solely by cytokeratin staining
- Must have undergone a modified radical mastectomy with
a level I and II axillary dissection (at least 10 nodes
examined) in past 8 months
- Surgical margins negative for invasive and noninvasive
ductal carcinoma
- No gross extracapsular disease or residual disease in
the axilla
- Microscopic extracapsular extension allowed
- Must have received chemotherapy with or without hormonal
therapy after mastectomy
- No more than 6 weeks since prior adjuvant chemotherapy
- Concurrent adjuvant chemotherapy allowed
- Concurrent tamoxifen allowed
STUDY
CONTACT
Lori J
Pierce, Ph: 734-936-7810
Southwest Oncology Group
University of Michigan Medical School
Thomas
Michael Pisansky, Chair, Ph: 507-284-4655
North Central Cancer Treatment Group
Stephen
Barrow Edge, Chair, Ph: 716-845-5789
American College of Surgeons Oncology Group
Robert
L Comis, Chair, Ph: 215-789-3645
Eastern Cooperative Oncology Group
Lawrence
J Solin, Chair, Ph: 215-662-7267
Radiation Therapy Oncology Group
Lawrence
Bruce Marks, Chair, Ph: 919-660-2127
Cancer and Leukemia Group B
|
Page
1of 2
Next Page
Top
Additional
Sections:
1 | 2
| 3 | 4
| 5 | 6
| 7 | 8
| 9 | 10
| 11 | 12
|
|
|

