|
 Home:
Educational
Supplement: Section 4
Home:
Educational
Supplement: Section 4
Section 4
Optimal
use of adjuvant tamoxifen and ovarian ablation
OVERVIEW
The International
Breast Cancer Overview has clearly demonstrated that adjuvant ovarian
ablation significantly reduces mortality in premenopausal patients
and that tamoxifen reduces mortality in both pre- and postmenopausal
women with ER-positive tumors. Current clinical trials are addressing
a number of important issues related to these two key interventions
, including the impact of combining these therapies with or without
chemotherapy. While five years is the accepted current standard
duration of adjuvant tamoxifen, randomized trials are evaluating
more prolonged treatment or sequencing tamoxifen with aromatase
inhibitors. Other SERMS are also being evaluated in various clinical
settings including the STAR trial, which compares raloxifene to
tamoxifen in high-risk women.
HISTORY OF
OVARIAN ABLATION
It is just
over 100 years since George Beatson described the dramatic responses
of three women with advanced breast cancer to surgical castration.
This became standard treatment for premenopausal women with advanced
breast cancer, and provided the first approach to trials of adjuvant
systemic therapy by pioneers Dr Cole (Manchester ) and Dr Nissen-Meyer
(Oslo). Unfortunately, they were underpowered statistically, and,
although they suggested that relapse-free survival could be prolonged,
it was not until the first world overview that we could confidently
conclude that such an approach improved survival.
-Michael
Baum, ChM, FRCS; Joan Houghton, BSc Br
Med J 1999;319:568-571.
15-YEAR
BENEFITS OF TAMOXIFEN
In receptor-positive
disease, the effects of five years of tamoxifen were very impressive
in preventing approximately 40% of recurrences within the first
10 years. We now have 15-year follow - up on these trials, and these
differences in recurrence and in survival are as big at year 15
as they we re at year 10. These are durable differences .
-
Richard Peto, FRS
SUSTAINED
ACTION OF TAMOXIFEN
The sustained
biological action of tamoxifen seems to last for many years after
it is no longer being administered. We have recently published a
potential explanation. After tumor cells are retransplanted into
animals for five years, tamoxifen stimulates growth, and no treatment
results in sluggish tumor growth. But when you treat with estrogen,
the tumors melt away. Physiological levels of estrogen attack the
supersensitized tumors, creating an apoptotic mechanism killing
these cells. This could explain how long-term adjuvant tamoxifenworks,
because on stopping around five years, the woman's own estrogen
now comes back and seeks out these supersensitized micrometastatic
breast cancer cells consolidating the beneficial effects of tamoxifen
with a second antitumor action.
-V
Craig Jordan, PhD, DSc
OVERVIEW
DATA ON OVARIAN ABLATION
The overview
data is from older studies and the number of patients in the ovarian
ablation trials are few, compared to the number in tamoxifen trials
or chemotherapy trials. There were several thousand patients in
the 60s and 70s, randomized to ovarian ablation or not, at first
- mainly in the absence of any chemotherapy. In some of the later
trials, chemotherapy plus ovarian ablation versus the same chemotherpy
alone was studied.
And in fact,
there are very clear data that ovarian ablation is beneficial compared
to no systemic treatment - with improvement, much like what was
seen with the chemotherapies of that day: CMF, AC and so on. And
because the studies are so old, the receptor status wasn't known
in many of the patients. In the later trials, you can see that added
to chemotherapy, ovarian ablation isn't quite as clearly significantly
effective, although it tends to add something to chemotherapy. It
may be that with chemotherapy some of these patients become amenorrheic
so that adding ovarian ablation doesn't do as much.
-Kathleen
Pritchard, MD
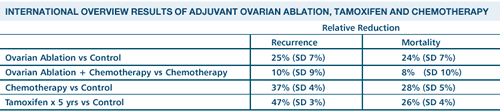
Lancet 1996;348:1189-1196.
Abstract
Lancet 1998;353:930-942. Abstract
| STUDY
OF TAMOXIFEN AND RALOXIFENE (STAR) FOR THE PREVENTION OF BREAST
CANCER Protocol
PROTOCOL
ID: : NSABP P-2
PROJECTED
ACCRUAL: Approximately 22,000 patients will be accrued
to this trial.
|
|

OBJECTIVES
- Determine whether raloxifene is more or less effective
than tamoxifen in significantly reducing the incidence rate
of invasive breast cancer in postmenopausal women.
- Evaluate the effects of tamoxifen and raloxifene on the
incidence of intraductal carcinoma in situ, lobular carcinoma
in situ, endometrial cancer, ischemic heart disease, fractures
of the hip and spine, or Colles' fractures of the wrist.
- Evaluate the toxic effects of these regimens in these
participants.
- Determine the effect of these regimens on the quality
of life of these participants.
PARTICIPATION
CRITERIA
- Postmenopausal women at increased risk for developing
invasive breast cancer
- Histologically confirmed lobular carcinoma in situ treated
by local excision only OR at least 1.66% probability of
invasive breast cancer within 5 years using Breast Cancer
Risk Assessment Profile
- No clinical evidence of malignancy on physical exam within
180 days
- No evidence of suspicious or malignant disease on bilateral
mammogram within the past year
- No bilateral or unilateral prophylactic mastectomy
- No prior invasive breast cancer or intraductal carcinoma
in situ
STUDY
CONTACT
Norman
Wolmark, Chair, Ph: 412-359-3336 National Surgical Adjuvant
Breast and Bowel Project Allegheny General Hospital, Pennsylvania
|
NSABP P-2:
THE STAR TRIAL
There is
a great deal of enthusiasm for this trial, and an enormous amount
of credit has to go to the NSABP members who are committed to moving
the state-of-the-art forward. That's their primary commitment, and
it was the primary commitment of the 13,388 selfless and courageous
women who entered the P-1 trial. And I believe that there will be
22,000 more women out there who will enter P-2.
-Norman Wolmark, MD
RALOXIFENE
Raloxifene
has been studied in a completely different population, namely older
postmenopausal women selected for osteoporosis risk, and some data
suggest that women with decreased bone density are at decreased
breast cancer risk. The raloxifene data on breast cancer risk reduction
is promising, but right now for postmenopausal women who are at
increased risk, tamoxifen is clearly the drug of choice outside
of a clinical trial. Also, raloxifene is only for postmenopausal
women; there are no safety data in premenopausal women.
-Monica
Morrow, MD
NSABP P-1:
THE BREAST CANCER PREVENTION TRIAL
... we consider
it highly inappropriate to not offer tamoxifen to women who are
similar to those in the P-1 study and who may benefit from its use
as a breast cancer preventive agent.
-Bernard
Fisher, MD et al. J Natl Cancer Inst 1998;90(18):1371-1388.
In my view,
both the certainty and the magnitude of the effect of tamoxifen
on the prevention of breast cancer are remarkable aspects of the
P-1 findings. In more than three decades of research, I have rarely,
if ever, observed such a major response to a breast cancer therapeutic
agent.
-Bernard
Fisher, MD J Clin Oncol 1999;17(5):1632-1639.
NSABP P-1
PROVIDES PROOF OF PRINCIPLE FOR CHEMOPREVENTION
The BCPT
has achieved an extraordinary advance for the control of breast
cancer and has provided the proof of principle of the chemopre-vention
approach. It is now established that human cancer can be prevented
with a pharmacologic intervention. Why did the BCPT succeed, whereas
many other phase III trials, particularly those involving ß-carotene,
have not achieved positive results? The answer seems to relate largely
to strength of rationale, which for the BCPT involved tamoxifen's
therapeutic efficacy, secondary phase III clinical findings (involving
contralateral breast cancer), and mechanistic/molecular-targeting
study.
-Scott
M Lippman, MD, Powel H Brown, MD, PhD J Natl Cancer Inst 1999;91(21):1809-1819.
| PHASE
III STUDY OF PROLONGED ADJUVANT TAMOXIFEN FOR CURATIVELY TREATED
BREAST CANCER Protocol
PROTOCOL
IDS: : UKCCCR-ATLAS, EU-96064
PROJECTED
ACCRUAL: Approximately 20,000 patients will be accrued.
|
|
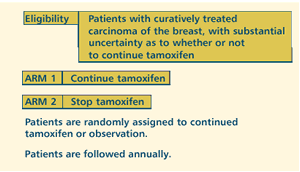
OBJECTIVES
- Assess the balance of risks and benefits in prolonging
the duration of adjuvant tamoxifen by at least five years
in patients with curatively treated breast cancer who have
already had about five years of adjuvant tamoxifen.
PARTICIPATION
CRITERIA
- Curatively treated carcinoma of the breast
- Must be substantial uncertainty as to whether or not to
continue tamoxifen (i.e., no clear indication or definite
contraindication to further treatment with tamoxifen)
- Currently taking adjuvant tamoxifen
STUDY
CONTACT
Christopher
J Williams, Chair, Ph: +44-1865-226628 United Kingdom Coordinating
Committee on Cancer Research Oxford, England, United Kingdom
|
| PHASE
III RANDOMIZED ADJUVANT STUDY OF TAMOXIFEN IN WOMEN WITH EARLY
BREAST CANCER Protocol
PROTOCOL
IDS: : CRC-TU-ATTOM, EU-98042
PROJECTED
ACCRUAL: A total of 8,000-20,000 patients will be accrued
into this study.
|
|

OBJECTIVES
- Compare the disease-free and overall survival of women
with early breast cancer who are randomized to stop adjuvant
tamoxifen with those randomized to continue for at least
5 extra years.
PARTICIPATION
CRITERIA
- Pre- or postmenopausal
- ER and/or PR-positive, -negative or unknown
- Histologically confirmed breast carcinoma that has been
completely excised
- Clinically relapse-free
- Must have completed at least two years of adjuvant therapy
with tamoxifen for early breast cancer AND no clear indication
for or against receiving further tamoxifen
- No significant endometrial hyperplasia
- No patients with negligibly low risk of breast cancer
death
STUDY
CONTACT
David
J Kerr, Ph: 0121-414-3802 Cancer Research Campaign Trials
Unit: University of Birmingham Birmingham, England, United
Kingdom
|
Page
1of 2
Next Page
Top
Additional
Sections:
1 | 2
| 3 | 4
| 5 | 6
| 7 | 8
| 9 | 10
| 11 | 12
|
|
|

