|
 Home:
Educational
Supplement: Section 8
Home:
Educational
Supplement: Section 8
Section 8
Herceptin
® (trastuzumab) as adjuvant therapy
OVERVIEW
Randomized trial
data from the advanced disease setting has demonstrated that in
women with HER2 overexpressing breast cancers, the combination of
Herceptin with chemotherapy - using either doxorubicin-cyclophosphamide
or paclitaxel - results in improved progression-free and overall
survival compared to the same chemotherapy given without Herceptin.
These encouraging results have led to a new generation of adjuvant
trials evaluating a variety of chemotherapeutic regimens combined
with Herceptin.
TARGETED SYSTEMIC
THERAPY
Herceptin
is clearly one of the major triumphs of the last few years. It's
exciting on many levels - largely because of the clinical benefit
but also because of the way it opens up a new era of science and
integration into the clinic...
...Following
on the heels of Herceptin, we are all very enthused about the kinase
inhibitors - agents like Iressa® (ZD 1839) - which will be,
to a greater or lesser extent, HER-targeting drugs, acting against
the epidermal growth factor receptor or members of the EGFR family.
And that may have an impact either as single agent therapy or, arguably,
in combination with conventional chemotherapy, just the way that
Herceptin has had an impact. Also, as we get our act together regarding
microarray assays and gene expression, we are going to begin to
separate patients in a way that conventional light microscopy does
not allow. I'm optimistic that this information will allow for better
treatment of patients, and maybe even allow us to select specific
therapies for specific patients. Look at the benefit of tamoxifen
in the Overview. Is that because tamoxifen is a powerful drug, or
because we select a subset of patients - ER-positive patients -
where we demonstrate a large benefit?
-Clifford
Hudis, MD
PHASE
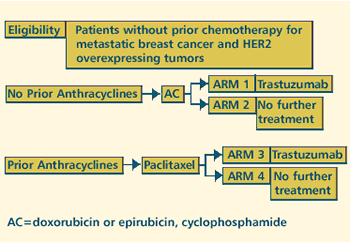
III RANDOMIZED STUDY OF CHEMOTHERAPY WITH VS WITHOUT MONOCLONAL
ANTIBODY HER2 IN WOMEN WITH METASTATIC BREAST CANCER OVEREXPRESSING
HER2/NEU AND PREVIOUSLY UNTREATED WITH CYTOTOXIC CHEMOTHERAPY
(closed to accrual) Protocol
PROTOCOL IDS: GENENTECH-HO648G; NCI-V95-0714 |
|
|
PHASE
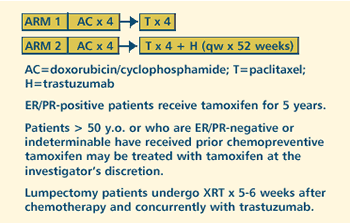
III RANDOMIZED STUDY OF DOXORUBICIN AND CYCLOPHOSPHAMIDE FOLLOWED
BY PACLITAXEL WITH OR WITHOUT TRASTUZUMAB (HERCEPTIN) IN WOMEN
WITH NODE-POSITIVE BREAST CANCER THAT OVEREXPRESSES HER2 Protocol
PROTOCOL
ID: NSABP
B-31
PROJECTED
ACCRUAL: A
total of 1,000-2,700 patients will be accrued within 4.75
years.N0-1, M0)
|
|
OBJECTIVES
- Compare the cardiotoxicity of doxorubicin and cyclophosphamide
followed by paclitaxel with or without trastuzumab (Herceptin)
in patients with operable, node-positive breast cancer that
overexpresses HER2.
- Compare the effect of these regimens, with or without
tamoxifen, on disease-free and overall survival.
PARTICIPATION
CRITERIA
- Invasive adenocarcinoma, stage IIA, IIB or IIIA (T1-3,
N0-1, M0)
- Clinically confined to the breast and ipsilateral axilla
- At least 1 axillary node histologically positive
- No lymph nodes clinically fixed to each other or to other
structures (N2 disease)
- HER2 strongly positive (3+ by immunostain or FISH). One
third or more invasive tumor cells must stain positive.
Submission of tumor block required
- Must have undergone ALND and either mastectomy OR lumpectomy.
SND allowed, if followed by ALND
- No bilateral malignancy. No contralateral mass or mammographic
abnormality unless proven benign
- No suspicious palpable nodes in the contralateral axilla,
supraclavicular or infraclavicular unless histologically
proven not to be involved with tumor
- No ulceration, erythema, infiltration of the skin or chest
wall, peau d'orange, or skin edema. Tethering or dimpling
of the skin or nipple inversion allowed
- No concurrent hormonal therapy, SERM therapy, or XRT (except
as specified in study)
STUDY
CONTACT
Edward
H. Romond, Chair, Ph: 859-323-8043 National Surgical Adjuvant
Breast and Bowel Project
|
SURVIVAL
ADVANTAGE IN METASTATIC DISEASE
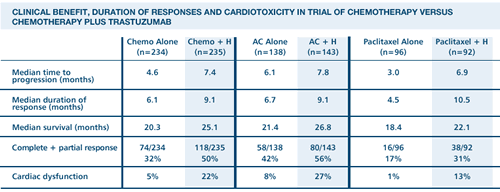
We found
that trastuzumab-based combination therapy was effective in that
it reduced the relative risk of death by 20 percent at a median
follow-up of 30 months. Few studies of metastatic breast cancer
have demonstrated a survival advantage of this magnitude in association
with the addition of a single agent. ...Given the extremely poor
prognosis of patients with HER2-positive metastatic breast cancer,
the cardiotoxicity of trastuzumab must be weighed against its potential
clinical benefit.
We recommend
a cautious approach to the use of trastuzumab in patients who have
previously received anthracyclines and in those who are currently
receiving anthracyclines. The adjuvant (postoperative) use of trastuzumab
will be an important research topic, but since many patients with
early-stage breast cancer can be cured by surgery and radiotherapy,
the cardiotoxicity of trastuzumab will be a critical consideration.
In this context, the risks of trastuzumab will necessitate great
caution in its use, especially when it is combined with an anthracycline.
Indeed, one large upcoming trial of adjuvant trastuzumab will evaluate
a nonanthracycline-based regimen for this reason.
In this context,
the risks of trastuzumab will necessitate great caution in its use,
especially when it is combined with an anthracycline. Indeed, one
large upcoming trial of adjuvant trastuzumab will evaluate a nonanthracycline-based
regimen for this reason.
-
Dennis J Slamon, MD, PhD et al.
N Engl J Med 2001;344(11):783-792.
TRASTUZUMAB
AS ADJUVANT THERAPY
There is
a powerful emotional and intellectual appeal to translate the survival
gains from the use of trastuzumab in the advanced-disease setting
to the adjuvant treatment of HER2-overexpressing breast cancer,
particularly with those at greatest risk of recurrence and death.
The undetermined
long-term risk of cardiac problems from trastuzumab, however, demands
caution, and use of the agent as adjuvant therapy is best restricted
to participation in the several clinical trials that are now underway.
Eligibility for these trials generally require tumors to have IHC
3+ or FISH-positive HER2 results and a pretreatment EF > 50%.
-
John Horton, MB, ChB, FACP
Cancer Control 2001;8(1):103-110.
| PHASE
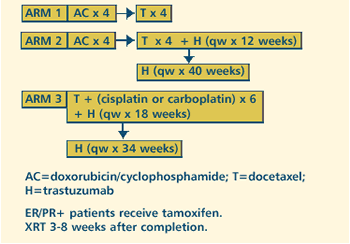
III RANDOMIZED STUDY OF ADJUVANT DOXORUBICIN, CYCLOPHOSPHAMIDE,
AND DOCETAXEL WITH OR WITHOUT TRASTUZUMAB (HERCEPTIN) VERSUS
TRASTUZUMAB, DOCETAXEL, AND EITHER CARBOPLATIN OR CISPLATIN
IN WOMEN WITH HER2-NEU-EXPRESSING NODE-POSITIVE OR HIGH-RISK
NODE-NEGATIVE OPERABLE BREAST CANCER Protocol
PROTOCOL
IDS: UCLA-010200601,
NCI-G01-1978, AVENTIS-TAX-GMA-302, BCIRG-006
PROJECTED
ACCRUAL: A
total of 3,150 patients (1,050 per treatment arm) will be
accrued within 2.5 years.
|
|
OBJECTIVES
- Compare disease-free survival in women with HER2- neu-expressing,
node+ or high-risk node-negative operable breast cancer
treated with adjuvant doxorubicin, cyclophosphamide, and
docetaxel with or without trastuzumab versus trastuzumab,
docetaxel, and either
- Compare overall survival of patients.
- Compare the toxic effects (including cardiac).
- Compare quality of life of patients treated with these
regimens
- Compare pathologic, molecular markets for predicting efficacy.
- Compare peripheral levels of shed HER2 ECD with FISH in
predicting outcome.
PARTICIPATION
CRITERIA
- 18-70 years old
- Histologically confirmed breast cancer (T1-3, N0-1, M0)
- Node+ disease OR high-risk node-disease with > 1 of the
following: tumor size > 2 cm, ER- and/or PR-negative, histologic
and/or nuclear grade 2-3
- No bilateral invasive breast cancer
- HER2-neu gene amplification by FISH
- Mastectomy or BCT AND ALN assessment within the past 60
days
- Clear margins histologically
STUDY
CONTACT
Linnea
Chap, Chair, Ph: 310-206-6144 Jonsson Comprehensive Cancer
Center, UCLA
|
HER2 ASSESSMENT
Reliable
detection of HER2 over expression is important for the success of
trastuzumab (Herceptin®) therapy. Several methods are available
for measuring HER2 expression at the DNA, RNA or protein level .
The method most frequently employed is immunohistochemical (IHC)
detection of the HER2 receptor in paraffin sections. Advantages
include the precise localization of the HER2 protein, the availability
of paraffin material and the ease of the procedure. However, IHC
can be influenced by the sensitivity/specificity of the antibody,
tissue treatment and, in particular, subjective assessment. These
disadvantages do not exist in the detection of gene amplification
by fluorescence in situhybridization (FISH) or polymerase chain
reaction. However, FISH requires expensive equipment that is not
widely available in pathology laboratories. Another approach quantitates
shed HER2 antigen in the serum by an enzyme-linked immunosorbent
assay. The key advantage of this method is the ease of sampling
blood, however, serum HER2 concentrations do not accurately reflect
the tumor status. Furthermore, this method does not register single-cell
expression, which is important for therapeutic decision-making.
For routine diagnostics, the combination of IHC and FISH is useful.
In addition, to improving the accuracy and comparability of HER2
assays, these optimized protocols may further enhance the efficacy
of trastuzumab therapy by selecting those patients most likely to
respond .
-Gerhard Schaller, MD et al.
Ann
Oncol 2001;(Suppl 1):S97-S100.
CLINICAL
TRIALS OF ADJUVANT TRASTUZUMAB
For HER2/neu-overexpressing
breast cancer patients, the adjuvant use of trastuzumab will become
paramount; therefore, it must be evaluated in a randomized controlled
trial. There is disagreement regarding the design of such a trial,
largely because of the ubiquitous use of anthracyclines in the adjuvant
setting and the opposing necessity of avoiding anthracycline plus
trastuzumab combinations. Combination index values for various chemotherapeutic
drugs in combination with trastuzumab demonstrate dramatic synergistic
interactions with the platinum agents and with docetaxel (Taxotere;
Aventis Pharmaceuticals, Inc, Parsippany, NJ). The greatest level
of synergy has been demonstrated with the triple-drug combination
of docetaxel, platinum, and trastuzumab in which synergy is demonstrated,
even at low doses. The adjuvant trial design for the Breast Cancer
International Research Group uses a control arm of doxorubicin/cyclophosphamide
for four cycles followed by docetaxel for four cycles and the second
arm contains the addition of trastuzumab to the taxane sequence.
The third arm, a non-anthracycline-containing regimen, contains
docetaxel, a platinum agent (either cisplatin or carboplatin), and
trastuzumab. The rationale for the selection of this three-drug
regimen is based on the biology of the system and preclinical and
clinical findings that demonstrate a high potential for clinical
synergy.
-Dennis
Slamon, MD, PhD, Mark Pegram, MD
Semin Oncol 2001;28(Suppl3):13-19.
| PHASE
III RANDOMIZED STUDY OF DOXORUBICIN AND CYCLOPHOSPHAMIDE WITH
OR WITHOUT DEXRAZOXANE, FOLLOWED BY PACLITAXEL WITH OR WITHOUT
TRASTUZUMAB (HERCEPTIN), FOLLOWED BY SURGERY AND RADIOTHERAPY
WITH OR WITHOUT TRASTUZUMAB IN WOMEN WITH HER-2+ STAGE IIIA
OR IIIB OR REGIONAL STAGE IV BREAST CANCER Protocol
PROTOCOL
IDS: CLB-49808,
CTSU
PROJECTED
ACCRUAL: A
total of 396 patients will be accrued within 4 years.
|
|
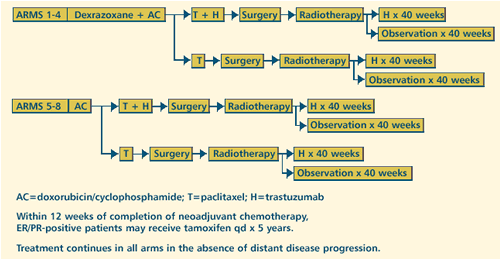
OBJECTIVES
- Determine the time to locoregional recurrence, time to
completion of treatment, and overall survival in women with
HER2+ stage IIIA or IIIB or regional stage IV breast cancer
treated with doxorubicin and cyclophosphamide (AC) with
or without dexrazoxane, followed by paclitaxel with or without
trastuzumab (Herceptin), followed by surgery and radiotherapy
with or without trastuzumab.
- Determine whether addition of trastuzumab to paclitaxel
therapy improves response at 24 weeks of therapy.
- Determine whether addition of trastuzumab to paclitaxel
therapy increases the rate of cardiotoxicity.
- Determine whether addition of dexrazoxane to AC compromises
response.
- Determine whether addition of dexrazoxane to AC reduces
the rate of cardiotoxicity.
- Determine whether long-term trastuzumab after local therapy
improves disease-free survival.
- Determine whether long-term trastuzumab after local therapy
increases the rate of cardiotoxicity.
- Determine the occurrence of any grade 3 or higher toxicity,
second malignancies, acute myelogenous leukemia, or myelodysplastic
syndrome.
- Determine the eventual rate of breast conservation in
those patients considered candidates for breast conservation
prior to neoadjuvant therapy.
- Determine the clinical response after AC with or without
dexrazoxane and the clinical/mammographic/ultrasound response
after paclitaxel with or without trastuzumab, compared to
the pathologic response at definitive surgery.
PARTICIPATION
CRITERIA
- 18 years and older
- Histologically confirmed primary infiltrating adenocarcinoma
of the breast confirmed by core needle biopsy or incisional
biopsy
- Amplification of HER2 by FISH OR IHC 3+
- Staging criteria after complete clinical and radiographic
staging:
- T3, N1, M0 OR
- Any T, N2 or N3, M0 OR
- T4, any N, M0 including clinical or pathological inflammatory
disease OR
- regional stage IV disease with supraclavicular or infraclavicular
lymph nodes as only site of metastasis
- Measurable or evaluable disease
- Prior DCIS of the ipsilateral breast allowed if treated
with excision only
- Metaplastic carcinoma allowed
- Synchronous bilateral primary disease allowed (provided
at least one cancer meets staging criteria)
- No dermal lymphatic involvement with clinical inflammatory
changes
STUDY
CONTACT
Mark L.
Graham, Chair, Ph: 919-859-6631 Cancer and Leukemia Group
B
|
| PHASE
III RANDOMIZED STUDY OF DOXORUBICIN PLUS CYCLOPHOSPHAMIDE FOLLOWED
BY PACLITAXEL WITH OR WITHOUT TRASTUZUMAB (HERCEPTIN) IN PATIENTS
WITH HER-2 OVEREXPRESSING BREAST CANCER Protocol
PROTOCOL
IDS: NCCTG-N9831,
GUMC-00224
PROJECTED
ACCRUAL: A
total of 3,000 patients (1,000 per treatment arm) will be
accrued over 4.5 years
|
|
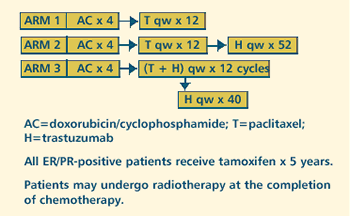
OBJECTIVES
- Compare the disease-free survival of patients with HER-2
overexpressing breast cancer when treated with doxorubicin
plus cyclophosphamide followed by paclitaxel with or without
trastuzumab (Herceptin).
- Compare the cardiotoxicities of these treatments.
- Compare the overall survival of these patients when treated
with one of these regimens.
- Determine whether higher levels of shed extracellular
domain or autoantibodies to HER-2 and HER-1 measured in
the serum prior to treatment are prognostic for disease-free
and overall survival.
- Determine the concordance of HER-2 overexpression with
disease-free and overall survival in this patient population.
PARTICIPATION
CRITERIA
- Menopausal status: Any status
- Histologically confirmed adenocarcinoma of the breast
with one or more positive lymph nodes (T1-3, pN1-2, M0)
- No cN2 disease allowed
- Metaplastic carcinoma and pN2 disease allowed
- HER-2/neu 3+ (0-2+ allowed if FISH+ assay)
- No locally advanced (T4) tumors.
- No dermal lymphatic involvement without clinical inflammatory
changes
- No bilateral invasive carcinoma or ductal carcinoma in
situ
- No gross or microscopic disease at margins
- No concurrent hormonal therapy or raloxifene
- No more than four weeks of prior tamoxifen allowed
- No prior radiotherapy for breast cancer
STUDY
CONTACT
Edith
A. Perez, Chair, Ph: 904-953-7283
North Central Cancer Treatment Group
Robert
L. Comis, Chair Ph: 215-789-3645
Eastern Cooperative Oncology Group
Peter
A. Kaufman, Chair Ph: 603-650-6700
Cancer
and Leukemia Group B
Silvana
Martino, Chair Ph: 310-998-3961
Southwest Oncology Group
|
TRASTUZUMAB-ASSOCIATED
CARDIOTOXICITY
A problem
in considering trastuzumab as adjuvant therapy is the unexpected
risk of developing cardiac dysfunction, particularly when combined
with the anthracycline doxorubicin. This would require careful monitoring
in patients with early breast cancer who have potentially curable
disease.
-
Joseph A Sparano, MD
Semin Oncol 2001;(1Suppl 3):20-27.
ADJUVANT
THERAPY AND CARDIOTOXICITY
Cardiac toxicity
was an unexpected side effect of trastuzumab treatment in the pivotal
trials that led to its approval. ..... The etiology of trastuzumab-associated
cardiac dysfunction is unknown, although its dependence on concurrent
or prior doxorubicin exposure suggests a common pathophysiologic
basis with anthracycline-induced myocardial injury. A number of
trials are in progress to evaluate the efficacy and safety of trastuzumab
in patients with early stage disease and that will investigate novel
strategies to circumvent this serious toxicity.
. . . In
an attempt to avoid any potential cardiotoxicity following administration
of trastuzumab plus doxorubicin, other therapeutic combinations
are being considered. These options include trastuzumab plus CMF,
epirubicin, liposomal doxorubicin, taxane-cisplatin (or carboplatin)
or taxane-vinorelbine.
. . . The
humanized anti-HER2 monoclonal antibody trastuzumab is an attractive
drug for adjuvant therapy because of its prolongation of survival
in metastatic disease, lack of cross-resistance to other agents,
and low incidence of toxicity. In order to answer questions relating
to the issue of cardiotoxicity following administration of a combination
of trastuzumab and doxorubicin, a number of trials are underway
in both metastatic patients and in the adjuvant setting. In addition,
it is important to consider non-anthracycline combinations. Finally,
the important issue of whether trastuzumab may confer a survival
benefit to tamoxifen treatment in a number of trials, is currently
being considered.
-
Ian Smith, MD
Ann Oncol 2001;(Suppl 1):S75-S79.
| PHASE
III RANDOMIZED STUDY OF PACLITAXEL VIA ONE HOUR INFUSION EVERY
WEEK VERSUS THREE HOUR INFUSION EVERY 3 WEEKS WITH OR WITHOUT
TRASTUZUMAB (HERCEPTIN) IN PATIENTS WITH INOPERABLE, RECURRENT,
OR METASTATIC BREAST CANCER WITH OR WITHOUT OVEREXPRESSION OF
HER2-NEU. Protocol
PROTOCOL
IDS: CLB
9840, CTSU
PROJECTED
ACCRUAL: A
total of 580 patients will be accrued within 3 years.
|
|
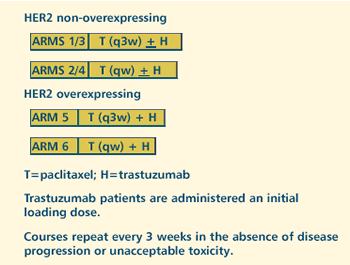
OBJECTIVES
- Compare the response rate in patients with inoperable,
recurrent, or metastatic breast cancer treated with paclitaxel
via one hour infusion every week vs 3 hour infusion every
3 weeks, regardless of HER2-Neu status and assignment to
trastuzumab.
- Compare the response rate and quality of life in patients
with HER2-Neu non-overexpressing disease treated with 1
of these 2 paclitaxel regimens with or without trastuzumab.
- Correlate amplification and overexpression of the growth
factor receptor ErbB2 by immunohistochemistry and fluorescent
in situ hybridization (FISH) with response rate, time to
progression, and overall survival in patients treated with
1 of these 2 paclitaxel regimens with or with out trastuzumab.
- Correlate ErbB2 shed extracellular domain (ECD) with response
rate, time to progression, and overall survival in patients
treated with 1 of these 2 paclitaxel regimens with or without
trastuzumab.
- Assess the patterns of ErbB2/ECD after treatment and upon
relapse.
- Compare the time to progression and survival of patients
with HER2-Neu overexpressing disease treated with 1 of these
2 paclitaxel regimens with trastuzumab.
- Compare the time to progression and survival in patients
with HER2-Neu nonoverexpressing disease treated with 1 of
these 2 paclitaxel regimens with or without trastuzumab.
- Compare the cardiac toxicity of these regimens as measured
by changes in left ventricular ejection fraction from baseline
to follow up measurements in these patients.
PARTICIPATION
CRITERIA
- 18 years and over
- Histologically proven inoperable, recurrent, or metastatic
adenocarcinoma of the breast
- Known HER2-neu status
- Measurable disease
STUDY
CONTACT
Andrew
D. Seidman, Chair, Ph: 212-636-5875 Cancer and Leukemia Group
B
|
INTEGRATING
TRASTUZUMAB INTO THE ADJUVANT SETTING
…the
significant cardiac toxicity observed with trastuzumab-anthracycline
combinations has led to two main strategies for integrating trastuzumab
in the adjuvant setting: (1) addition of trastuzumab to mostly anthracycline-based
programs (sequential approach); and (2) biology-oriented strategy
based on synergism between trastuzumab and chemotherapy agents.
Large-scale clinical research programs are presently being developed
and will create a challenge for clinical researchers. The adequate
scientific hypothesis, related to the pivotal studies of trastuzumab
in the adjuvant setting, require large sample sizes (several thousand
patients) and a very strict selection of the patient population
(tumors amplifying the HER2 gene). Success in a timely fashion requires
global collaboration, dedication to high-standard clinical research,
and awareness of all available protocols by oncologists and patients
with breast cancer.
-Jean-Marc
Nabholtz, MD, Dennis Slamon, MD, PhD
Semin Oncol 2001;28(Suppl1):1-12.
HER2 REPRODUCIBILITY
Consensus
regarding the best methods, reagents, or cut-off points to define
HER2 status for determining trastuzumab responsivity has not yet
been reached. HER2 testing for other prognostic or predictive purposes,
e.g. to determine whether patients are likely to respond to other
agents, such as dose-intensive doxorubicin, may be less. Data from
the Cancer and Leukemia Group B trial 8541 (companion 8869) suggest
that, with proper controls in high-volume laboratories, many of
the available methods produce comparable results.
-Ann
Thor, MD
Ann Oncol 2001;(Suppl 1):S101-S107.
| PHASE
III RANDOMIZED STUDY OF FIRST-LINE TRASTUZUMAB (HERCEPTIN) ALONE
FOLLOWED BY COMBINATION TRASTUZUMAB AND PACLITAXEL VERSUS FIRST-LINE
COMBINATION TRASTUZUMAB AND PACLITAXEL IN WOMEN WITH HER2 OVEREXPRESSING
METASTATIC BREAST CANCER Protocol
PROTOCOL
IDS: SWS-SAKK-22/99,
EU-99028
PROJECTED
ACCRUAL: Approximately
170-250 patients.
|
|
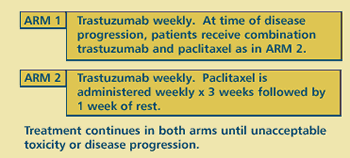
OBJECTIVES
- Compare efficacy and toxicity of first-line trastuzumab
alone followed at disease progression by the combination
of trastuzumab and paclitaxel versus first-line combination
of both drugs in women with HER2 overexpressing metastatic
breast cancer.
- Compare quality of life of these patients.
- Investigate the predictive value of serum HER2/neu ECD
levels on clinical outcome, the effects of trastuzumab on
estrogen receptor, and the association of immunoprofiles
of erbB-1, erbB-2, erbB-3, and erbB-4 with clinical outcome
in this patient population.
PARTICIPATION
CRITERIA
- Histologically confirmed HER2 overexpressing metastatic
breast carcinoma
- Clinically or radiologically measurable or evaluable disease
- Bidimensionally or unidimensionally measurable lesions
- No cumulative dose of doxorubicin greater than 240 mg/m2
- No cumulative dose of epirubicin greater than 360 mg/m2
- No prior taxanes
STUDY
CONTACT
Aron Goldhirsch,
Chair, Ph: 39-02-574-894-39 Swiss Institute for Applied Cancer
Research Istituto Europeo Di Oncologia Milano, Italy
|
| PHASE
II/III RANDOMIZED STUDY OF ANASTROZOLE WITH OR WITHOUT TRASTUZUMAB
(HERCEPTIN) IN POSTMENOPAUSAL WOMEN WITH HORMONE-RECEPTOR POSITIVE
HER2-OVEREXPRESSING METASTATIC BREASTCANCER Protocol
PROTOCOL
IDS: ROCH-BO16216,
GENENTECH-H2223g
PROJECTED
ACCRUAL: 202
patients (101 per arm) in 24 months.
|
|

OBJECTIVES
- Compare the efficacy with or without trastuzumab, in terms
of progression-free survival, in postmenopausal women with
hormone receptor-positive, HER2-overexpressing metastatic
breast cancer.
- Compare the safety profiles of these regimens.
- Compare the overall clinical benefit rate
- Compare the overall survival, duration of response and
two-year survival of patients treated with these regimens.
PARTICIPATION
CRITERIA
- Histologically or cytologically confirmed metastatic breast
cancer
- HER2 3+ by IHC or any degree of HER2 gene amplifications
(at least 2 fold) by FISH
- ER and/or PR receptor+ postmenopausal women
- No concurrent chemo or hormonal therapy, nor concurrent
radiotherapy or surgery to only known site of disease
STUDY
CONTACT
Bernd
Langer, Chair, Ph: 41-61-68-80638 Roche Global Development-Palo
Alto
|
Select
Publications
Top
Additional
Sections:
1 | 2
| 3 | 4
| 5 | 6
| 7 | 8
| 9 | 10
| 11 | 12
|
|
|

