|
 Home:
Educational
Supplement: Section 10
Home:
Educational
Supplement: Section 10
Section 10
Bisphosphonates
as adjuvant therapy
OVERVIEW
A number of
biologic effects in bone suggest that bisphosphonates have the potential
to retard or prevent the clinical onset of metastatic disease. Three
randomized adjuvant trials have yielded conflicting results on this
question, although the use of pamidronate is now considered standard
in the patients with known lytic bone metastases. A new generation
of adjuvant trials is now evaluating whether bisphosphonates will
reduce the rate of bone and nonbone metastases.
PRIOR ADJUVANT
TRIALS
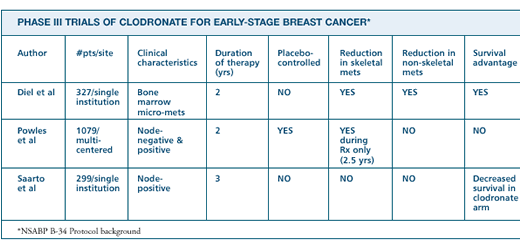
There are
results from three randomized trials of adjuvant clodronate. In
our study, patients receiving clodronate had fewer subsequent bone
and nonbone metastases. The largest study was done in Great Britain,
Canada and Scandinavia. In terms of methodology, it's the best,
because it was placebo-controlled and double-blinded. They found
a reduction in bone metastases but no significant effect on overall
survival. The third study was done in Finland and was not placebo-controlled.
In 300 node-positive patients, they observed no effect on the rate
of bone metastases and a negative effect on disease-free survival
and nonosseous metastases. No other bisphosphonate study has reported
this type of negative effect. In addition to different methodology,
the clear difference in the three studies is the selection of patients.
When we started
our study ten years ago, we selected patients with tumor cells in
the bone marrow, because we were convinced this was the best prognostic
factor for bone metastases. Today we know it's also a good prognostic
factor for nonbone metastases, because it reflects the early hematogenous
spread of breast cancer cells from the primary tumor. Clodronate
is not available in the United States, because several years ago,
the initial studies reported a number of leukemias that were eventually
demonstrated to be unrelated to clodronate. From that time it was
not pursued as an antiosteolytic drug in the United States. The
problem with the bisphosphonates is that the gastrointestinal absorption
rate is low, and in order to see an effect, you need a dose that
may cause side effects, particularly on the gastrointestinal tract.
I support the new NSABP clodronate study, because it's important
to confirm our data, but I don't know whether it was a good decision
to include all breast cancer patients as opposed to attempting to
select those most likely to develop bone metastases.
-Ingo
Diel, MD
MECHANISM
OF ACTION
We have a
large number of bisphosphonates either in the clinic or becoming
available, and their potency is increasing. Most data are from clodronate
and pamidronate - osteoclast inhibitors. Recently it was shown that
these non-nitrogen-containing bisphosphonates appear to have a different
mechanism of action at the cellular level than the nitrogen-containing
bisphosphonates.
In advanced
disease, we mostly use bisphosphonates to reduce complications in
conjunction with other standard treatments. We are looking to control
pain, hypercalcemia and fractures, and to reduce the requirements
for radiotherapy and surgery. In adjuvant studies, clodronate reduces
metastases in bone by a small amount, and in one study, there was
a significant reduction in visceral metastases and an improvement
in survival, but we need to do other studies to confirm this.
-Professor
Anthony Howell, FRCP
| PHASE
III RANDOMIZED STUDY OF ADJUVANT CLODRONATE WITH OR WITHOUT
SYSTEMIC CHEMOTHERAPY AND/OR TAMOXIFEN IN WOMEN WITH EARLY-STAGE
BREAST CANCER Protocol
PROTOCOL
IDS: NSABP-B-34,
CTSU
PROJECTED
ACCRUAL: A
total of 2,400 patients will be accrued for this study within
4 years.
|
|

OBJECTIVES
- Determine whether clodronate administered alone or in
addition to adjuvant chemotherapy and/or tamoxifen improves
disease-free survival in patients with early-stage breast
cancer.
- Determine whether clodronate reduces the incidence of
skeletal metastases and nonskeletal metastases.
- Determine whether clodronate improves overall and relapse-free
survival in these patients.
- Determine whether clodronate reduces the incidence of
skeletal morbidity (e.g., skeletal fractures, hypercalcemia,
skeletal pain, need for radiotherapy, spinal cord compression)
in these patients.
- Determine the relevance of serum markers of bone turnover
as a prognostic factor for the development of bone metastases
in these patients.
PARTICIPATION
CRITERIA
- Histologically proven invasive adenocarcinoma of the breast
Stage I or II (T1-3, N0-1, M0)
- No significant nonmalignant bone disease that is likely
to interfere with interpretation of bone X-rays
- Skeletal pain allowed only if bone scan and/or roentgenological
examination fails to disclose metastatic disease
- Suspicious findings must be confirmed as benign by X-ray,MRI
or biopsy
STUDY
CONTACT
Alexander
H. G. Paterson, Chair, Ph: 403-670-1707 National Surgical
Adjuvant Breast and Bowel Project
|
| PHASE
III RANDOMIZED STUDY OF ZOLEDRONATE AS ADJUVANT THERAPY IN PATIENTS
WITH STAGE I, II OR IIIA NONMETASTATIC BREAST CANCER Protocol
PROTOCOL
ID: :
SWOG-S9905
PROJECTED
ACCRUAL: A
total of 3,300 patients will be accrued for this study over
3.5 years.
|
|

OBJECTIVES
- Compare disease-free and overall survival in patients
with stage I, II or IIIA nonmetastatic breast cancer treated
with standard adjuvant therapy and zoledronate to those
treated with standard adjuvant therapy alone (observation
only).
- Assess whether zoledronate added to standard adjuvant
therapy influences the first site of recurrence in these
patients.
- Compare the first site of recurrence in PTHrP-positive
patients with the first site of recurrence in PTHrP-negative
patients in the group not receiving zoledronate.
- Explore whether treatment effects are different within
the PTHrP-positive and PTHrP-negative subsets.
PARTICIPATION
CRITERIA
- Age, menopausal status, hormone receptor status: Not specified
- Histologically confirmed stage I, II or IIIA primary invasive
adenocarcinoma of the breast. No metastatic disease
- Must have undergone modified radical mastectomy or BCT
plus either ALND or SLNB
- Prior or concurrent standard systemic adjuvant therapy
required
- Prior neoadjuvant chemotherapy allowed
- Combined hormonal/chemotherapy or hormonal therapy alone
allowed
- Concurrent radiotherapy allowed
STUDY
CONTACT
Charles
A. Coltman, Jr., Ph: 210-616-5580
Southwest Oncology Group
University of Colorado Cancer Center
Denver, Colorado
|
| PHASE
III RANDOMIZED STUDY OF ZOLEDRONATE, CALCIUM AND CHOLECALCIFEROL
(VITAMIN D) TO PREVENT BONE LOSS IN WOMEN WITH BREAST CANCER
RECEIVING ADJUVANT CHEMOTHERAPY Protocol
PROTOCOL
IDS: CLB-79809,
NCI-P01-0184
PROJECTED
ACCRUAL: Approximately
400 patients (200 per treatment arm) will be accrued for this
study within 24 months.
|
|

OBJECTIVES
- Compare the bone mineral density in the lumbar spine after
12 and 36 months of therapy with zoledronate, calcium, and
cholecalciferol (vitamin D) in women with breast cancer
receiving adjuvant chemotherapy.
PARTICIPATION
CRITERIA
- Age: 40 and over
- Premenopausal
- Histologically confirmed adenocarcinoma of the breast
by fine needle aspirate, biopsy (tru-cut, core, stereotactic),
lumpectomy or modified radical mastectomy
- Stage I-III (any T, any N, M0)
- Stage IV due solely to supraclavicular node involvement
allowed
- Plan to use adjuvant chemotherapy with or without tamoxifen
STUDY
CONTACT
Richard
L. Schilsky, Chair Ph: 773-834-3914 Cancer and Leukemia Group
B
|
Select
Publications
Top
Additional
Sections:
1 | 2
| 3 | 4
| 5 | 6
| 7 | 8
| 9 | 10
| 11 | 12
|
|
|

