|
 Home:
Educational
Supplement: Section 7
Home:
Educational
Supplement: Section 7
| PHASE
III STUDY OF DOXORUBICIN AND CYCLOPHOSPHAMIDE FOLLOWED BY PACLITAXEL
OR DOCETAXEL IN WOMEN WITH NODE-POSITIVE OR HIGH-RISK NODE-NEGATIVE
STAGE II OR IIIA BREAST CANCER Protocol
PROTOCOL
ID: E-1199
PROJECTED
ACCRUAL: A
total of 5,000 patients will be accrued within 1.27 years.
|
|
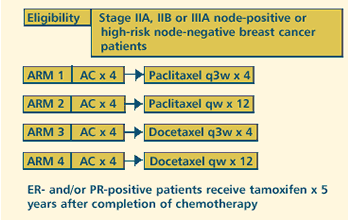
OBJECTIVES
- Compare the disease-free survival and overall survival
in patients with node-positive or high-risk node-negative
operable stage II or IIIA breast cancer treated with docetaxel
or paclitaxel after AC.
- Determine whether the weekly administration of paclitaxel
or docetaxel for 12 weeks improves disease-free survival
and overall survival when compared with the conventional
schedule of every 3 weeks for 4 courses after AC.
- Compare the toxic effects of docetaxel and paclitaxel
when administered weekly for 12 weeks versus every 3 weeks
for 4 courses in these patients.
- Compare the toxicity of paclitaxel administered every
3 weeks for 4 courses or weekly for 12 weeks to that of
docetaxel administered on the same schedules in these patients.
PARTICIPATION
CRITERIA
- 18 and over, any hormone receptor status
- Histologically confirmed operable stage IIA, IIB or IIIA
adenocarcinoma of the breast with histologically involved
lymph nodes OR high-risk node-negative disease
- Tumor at least 2.1 cm in diameter for node-negative
disease
- Bilateral breast disease allowed if at least 1 primary
tumor meets the criteria above
- Must have had at least 6 axillary lymph nodes removed
at dissection and at least one node positive OR sentinel
node biopsy negative for metastasis (SNLB+ allowed if enrolled
on American College of Surgery Trial Z0011 and have been
randomized to receive no axillary dissection)
- Tumor-free margins at least 1 mm for both invasive and
noninvasive carcinoma except for LCIS (< 1 mm allowed)
- Concurrent enrollment on ACS Z0010, Z0011 or NSABP B-32
allowed
STUDY
CONTACT
Joseph
A Sparano, Chair, Ph: 718-904-2555 Eastern Cooperative Oncology
Group Edith A Perez, Chair, Ph: 904-953-7283 North Central
Cancer Treatment Group Silvana Martino, Chair, Ph: 310-998-3961
Southwest Oncology Group Vicky Eileen Jones, Chair, Ph: 858-657-8710
Cancer and Leukemia Group B
|
| PHASE
IV STUDY OF EPOETIN ALFA IN WOMEN WITH STAGE I, II OR III BREAST
CANCER AND CHEMOTHERAPY-RELATEDANEMIA Protocol
PROTOCOL
IDS: UCLA-0011004,
NCI-G01-2002, ORTHO-PR-00-27-012, ORTHO-PR-01-27-003
PROJECTED
ACCRUAL: A
maximum of 2,500 patients will be accrued for this study.
|
OBJECTIVES
- Determine the effectiveness and safety of epoetin alfa
in patients receiving adjuvant chemotherapy for stage I,
II or III breast cancer.
- Determine the clinical outcomes in these patients receiving
this drug.
PARTICIPATION
CRITERIA
- Age: 18 and over
- Histologically or cytologically confirmed stage I, II
or III breast cancer
- Planned adjuvant anthracycline-based chemotherapy with
or without a taxane for 3-6 months
- Hemoglobin 10-14 g/dL (independent of transfusion)
- No anemia due to factors other than cancer/chemotherapy
(i.e., iron, cyanocobalamin or folate deficiency; hemolysis;
gastrointestinal bleeding; or myelodysplastic syndrome)
- At least 6 months since prior epoetin alfa or any investigational
forms of erythropoietin (e.g., gene-activated erythropoietin
or novel erythropoiesis-stimulating protein)
PROTOCOL
Patients
receive epoetin alfa subcutaneously once weekly for up to
24 weeks in the absence of unacceptable toxicity.
STUDY
CONTACT
John A
Glaspy, Chair Ph: 310-794-1274 Jonsson Comprehensive Cancer
Center, UCLA
|
| PHASE
III RANDOMIZED STUDY OF ADJUVANT DOXORUBICIN AND CYCLOPHOSPHAMIDE
FOLLOWED BY DOCETAXEL VERSUS DOXORUBICIN AND DOCETAXEL VERSUS
DOXORUBICIN, DOCETAXEL AND CYCLOPHOSPHAMIDE IN WOMEN WITH BREAST
CANCER AND POSITIVE AXILLARY LYMPH NODES Protocol
PROTOCOL
IDS:
NSABP B-30; CTSU
PROJECTED
ACCRUAL: A
total of 4,000 patients will be accrued within 3 years.
|
|
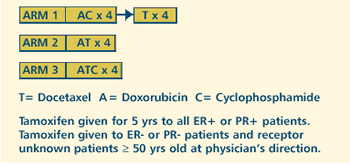
OBJECTIVES
- Compare the efficacy of adjuvant AC and docetaxel given
concurrently versus adjuvant AC followed by docetaxel, in
terms of overall survival and disease-free survival of women
with breast cancer and positive axillary lymph nodes.
- Compare the efficacy of adjuvant doxorubicin and docetaxel
versus regimens containing cyclophosphamide.
- Compare the toxic effects of these regimens.
- Compare the quality of life of these patients.
- Compare the differences in amenorrhea in premenopausal
women in each treatment arm and its relationship to symptoms,
quality of life, disease-free survival and overall survival.
PARTICIPATION
CRITERIA
- Age unspecified, hormone receptor status known
- Histologically proven invasive adenocarcinoma of the breast
confined to the breast and ipsilateral axilla on clinical
exam
- Stage I, II or IIIA (T1-3, N0-1, M0)
- At least one axillary lymph node with tumor on histologic
exam
- Sentinel node biopsy allowed if followed by axillary
dissection
- No suspicious palpable nodes in the contralateral axilla
or palpable supraclavicular or infraclavicular nodes,
unless proven on biopsy to not be involved with tumor
- No bilateral malignancy or mass in the opposite breast,
unless mass is histologically proven to be benign
- Must have undergone either a prior total mastectomy and
axillary dissection (modified radical mastectomy) OR prior
lumpectomy and axillary dissection
- Patients must receive radiotherapy after randomization
- Margins must be clear
- No N2 disease and/or any positive nonaxillary lymph nodes
- No metastatic disease by X-ray, MRI or biopsy
- Skeletal pain allowed if bone scan negative for metastases
STUDY
CONTACT
Sandra
M Swain, Chair, Ph: 301-496-4916 National Surgical Adjuvant
Breast and Bowel Project
|
| PHASE
III STUDY OF ADJUVANT EPIRUBICIN WITH OR WITHOUT DOCETAXEL AND
CONCURRENT OR SEQUENTIAL TAMOXIFEN IN POSTMENOPAUSAL WOMEN WITH
NODE-POSITIVE BREAST CANCER Protocol
PROTOCOL
IDS: ICCG-C/14/96,
EU-20040
PROJECTED
ACCRUAL: A
total of 800 patients will be accrued for this study.
|
|

OBJECTIVES
- Compare the impact of adjuvant epirubicin with or without
docetaxel and concurrent or sequential tamoxifen on time
to relapse and overall survival in postmenopausal women
with node-positive breast cancer.
- Compare the toxic effects of these regimens in this patient
population.
- Compare the quality of life in terms of shift in long-term
toxicity and differences in recuperation.
- Compare the incidence of thromboembolic events during
the first 9 months of study and the influence of such events
on compliance in women treated with these regimens.
PARTICIPATION
CRITERIA
- Histologically proven node-positive breast cancer
- Postmenopausal
- No distant metastases
STUDY
CONTACT
Raoul
C Coombes, Chair, Ph: +44 (0)20 8846 14 18 International Collaborative
Cancer Group Charing Cross Hospital London, England, United
Kingdom
|
| PHASE
III RANDOMIZED STUDY OF ADJUVANT CYCLOPHOSPHAMIDE, EPIRUBICIN
AND FLUOROURACIL VERSUS CYCLOPHOSPHAMIDE, EPIRUBICIN, FILGRASTIM
(G-CSF) AND EPOETIN ALFA FOLLOWED BY PACLITAXEL VERSUS CYCLOPHOSPHAMIDE
AND DOXORUBICIN FOLLOWED BY PACLITAXEL IN PREMENOPAUSAL OR EARLY
POSTMENOPAUSAL WOMEN WITH PREVIOUSLY RESECTED NODE-POSITIVE
OR HIGH-RISK NODE-NEGATIVE STAGE I-IIIA BREAST CANCER Protocol
PROTOCOL
IDS: CAN-NCIC-MA21,
AMGEN-CAN-NCIC-MA21, BMS-CAN-NCIC-MA21, JANSSEN-CAN-NCIC-MA21,
P-UPJOHN-CAN-NCIC-MA21
PROJECTED
ACCRUAL: A
total of 1,500 patients (500 per treatment arm) will be accrued
for this study within 3 years.
|
|
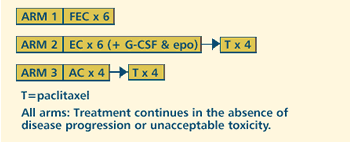
OBJECTIVES
- Compare the disease-free survival and overall survival
of premenopausal or early postmenopausal women with previously
resected node-positive or high-risk node-negative stage
I-IIIA breast cancer treated with cyclophosphamide, epirubicin
and fluorouracil vs cyclophosphamide, epirubicin, filgrastim
(G-CSF) and epoetin alfa followed by paclitaxel vs cyclophosphamide
and doxorubicin followed by paclitaxel.
- Compare the rate of toxic effects of these regimens.
- Compare the quality of life of patients treated with these
regimens.
PARTICIPATION
CRITERIA
- Age: 60 and under
- Histologically confirmed adenocarcinoma of the breast
that is potentially curable
- Stage I-IIIA (T0-4 (dermal involvement only), N0-2, M0)
- Axillary node-positive or high-risk node-negative
- Previously treated with total mastectomy and axillary
node dissection or partial mastectomy and axillary node
dissection with planned breast radiotherapy after completion
of study or sentinel node biopsy (if node-positive, must
undergo axillary node dissection)
- No prior immunotherapy, chemotherapy, radiotherapy or
hormonal therapy for breast cancer
STUDY
CONTACT
Margot
J Burnell, Chair, Ph: 506-648-6884 NCIC-Clinical Trials Group
|
| PHASE
III RANDOMIZED STUDY OF ADJUVANT INDUCTION CHEMOTHERAPY WITH
OR WITHOUT CYCLOPHOSPHAMIDE AND METHOTREXATE AS MAINTENANCE
CHEMOTHERAPY IN PATIENTS WITH STAGE I, II, OR III BREAST CANCER
Protocol
PROTOCOL
IDS: IBCSG-22-00,
EU-20119
PROJECTED
ACCRUAL: Approximately
1,330 patients will be accrued for this study within 5 years.
|
|
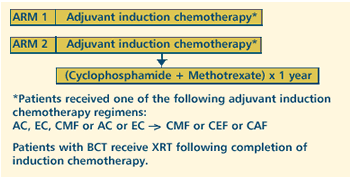
OBJECTIVES
- Determine the efficacy of adjuvant induction chemotherapy
with or without cyclophosphamide and methotrexate as maintenance
chemotherapy in patients with stage I, II or III breast
cancer.
- Compare the disease-free, overall and systemic disease-free
survival of patients treated with these regimens.
- Compare the toxic effects of these regimens in these patients.
- Compare the quality of life of patients treated with these
regimens.
PARTICIPATION
CRITERIA
- Pre-or postmenopausal
- Histologically confirmed stage I, II or III breast cancer
(T1-3, N0-1, M0. T4 disease with minimal dermal invasion
allowed)
- No distant metastases
- Prior mastectomy OR BCT within past 6 weeks
- Estrogen and progesterone receptor-negative
- No prior radiotherapy for breast cancer
STUDY
CONTACT
Marco
Colleoni, Chair, Ph: 039-2-57489439 International Breast Cancer
Study Group
|
| COMPANION
STUDY TO EVALUATE LATE CARDIAC EFFECTS IN WOMEN WITH NODE-NEGATIVE
BREAST CANCER RECEIVING ADJUVANT CHEMOTHERAPY ON PROTOCOL SWOG-8897
Protocol
PROTOCOL
ID: :
SWOG-9342
PROJECTED
ACCRUAL: A
total of 420 patients will be accrued for this study. After
initial accrual is completed, approximately 50 additional
patients will be accrued at 10 years.
|
OBJECTIVES
- Compare the frequency of subclinical congestive heart
failure by measuring resting MUGA at 5-8 and 10-11 years
after randomization in women receiving adjuvant chemotherapy
with cyclophosphamide, methotrexate and fluorouracil or
cyclophosphamide, doxorubicin and fluorouracil on protocol
SWOG-8897.
- Estimate the frequency of late cardiac effects (congestive
heart failure, cardiac ischemic events and clinical symptoms)
in these patients treated with these regimens.
- Monitor prospectively the incidence of annual cardiac
events between the fifth and tenth year after randomization
of these patients to these regimens.
PARTICIPATION
CRITERIA
- Age: 18 and over
- Women registered on Arm I, II, III or IV of protocol SWOG-8897
who have completed at least 1 course of assigned chemotherapy
- Completion of tamoxifen therapy not required
- Registration to current study required between 5.25-8
years or 10-11 years after randomization to protocol SWOG-8897
- Patients must be diagnosed disease-free with no prior
recurrence after registration on protocol SWOG-8897
PROTOCOL
The treating
physician completes patient cardiovascular and routine history
and physical examination questionnaires at baseline and yearly.
Patients undergo resting MUGA scans at 5-8 and 10-11 years
after registration on protocol SWOG-8897. The first scan must
be performed within 3 months prior to enrollment or within
1 month after registration on the current study, and the second
scan must be done in the tenth year of follow-up and within
3 months prior to enrollment or 1 month from the anniversary
of registration on the current study.
STUDY
CONTACT
Patricia
A Ganz, Chair, Ph: 310-206-1404 Southwest Oncology Group
|
| PROSPECTIVE
STUDY OF POTENTIAL FACTORS AFFECTING WEIGHT IN BREAST CANCER
PATIENTS RECEIVING ADJUVANT CHEMOTHERAPY Protocol
PROTOCOL
IDS: NCI-99-C-0026,
NCI-99-C-0020
PROJECTED
ACCRUAL: A
total of 140 patients will be accrued for this study within
1-2 years.
|
OBJECTIVES
I. Evaluate
the relative contributions of factors that may lead to weight
gain in breast cancer patients receiving adjuvant chemotherapy.
Factors examined include:
- Hormonal and growth factor status (follicle stimulating
hormone; total, bound and free estradiol; androgens; sex
hormone-binding globulin; thyroid hormones; prolactin;
insulin-like growth factors I and II; and plasma leptin).
- Factors affecting energy intake or expenditure (oral
intake, physical activity and resting metabolic rate).
- Psychological factors (depression and quality of life).
II.
Evaluate the effect of chemotherapy on hormonal and growth
factor status in these patients.
III. Assess
the impact of chemotherapy on bone marrow density in these
patients.
PARTICIPATION
CRITERIA
- Age: 18 to 80
- Menopausal status: Premenopausal or postmenopausal
- Histologically proven newly diagnosed stage I, II or resectable
IIIA primary breast cancer
- Scheduled to receive chemotherapy
- No prior oophorectomy
PROTOCOL
Data is
collected from women diagnosed with primary breast cancer
at 3 points (5 visits): (I) after breast cancer surgery, but
before chemotherapy begins (2 visits to NIH day hospital 1
week apart); (II) 2-3 weeks after chemotherapy has ended (2
visits, 1 week apart); and (III) 6 months after chemotherapy
has ended (1 visit). Tests conducted during these visits include
evaluation of blood for hormones, growth factors, and leptin;
body composition by DXA; visceral and subcutaneous abdominal
adipose tissue by an axial CT scan; and evaluation of resting
metabolic rate and daily energy expenditure by a single administration
of doubly labeled water at visits "a" and "b"
during data collection timepoints 1 and 2. Questionnaires
assessing epidemiologic risk factors for breast cancer, dietary
intake, physical activity, depression, and quality of life
are also administered at the three timepoints.
STUDY
CONTACT
Noreen
Aziz, Ph: 301-496-0598 Cancer Prevention Studies Branch Bethesda,
Maryland
|
| PHASE
III RANDOMIZED STUDY OF ADJUVANT CHEMOTHERAPY USING STANDARD
CYCLOPHOSPHAMIDE/ METHOTREXATE/FLUOROURACIL (CMF) OR DOXORUBICIN/CYCLOPHOSPHAMIDE
(AC) VERSUS ORAL CAPECITABINE IN ELDERLY WOMEN WITH OPERABLE
ADENOCARCINOMA OF THE BREAST (STUDY APPROVED, NOT YET ACTIVE)
Protocol
PROTOCOL
IDS: CLB-49907,
CTSU
PROJECTED
ACCRUAL: A
total of 600-1,800 patients (300-900 per treatment arm) will
be accrued for this study within 2-6 years.
|
|
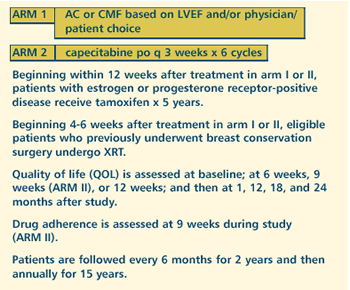
OBJECTIVES
- Compare the effectiveness of adjuvant chemotherapy using
standard CMF or AC vs oral capecitabine in terms of disease-free
and overall survival in elderly women with operable adenocarcinoma
of the breast.
- Compare the quality of life and physical functioning of
patients treated with these regimens.
- Compare the toxicity of these regimens in these patients.
- Evaluate the adherence of older patients to an oral chemotherapy
regimen.
ELIGIBILITY
- Postmenopausal women age 65 and over
- Histologically proven operable adenocarcinoma of the breast
- Stage IIA or IIIA disease ( T1-3, N1, M0 or T2, N0, M0
if primary lesion at least 3 cm)
- Hormone receptor status not specified
- Must have undergone 1 of the following within the past
12 weeks: Modified radical mastectomy OR lumpectomy with
axillary lymph node dissection or sentinel node biopsy.
Prior full axillary dissection required if positive sentinel
node(s)
- Any number of previously excised nodes allowed
- No prior chemotherapy for breast cancer
- No other concurrent chemotherapy or hormonal therapy
STUDY
CONTACT
Richard
L. Schilsky, Chair Ph: 773-834-3914 Cancer and Leukemia Group
B
|
Page
2 of 3
Previous | Next
Top
Additional
Sections:
1 | 2
| 3 | 4
| 5 | 6
| 7 | 8
| 9 | 10
| 11 | 12
|
|
|

