|
 Home:
Educational
Supplement: Section 4
Home:
Educational
Supplement: Section 4
DURATION
OF TAMOXIFEN THERAPY
Breast cancer
is a disease with at least a 20-year natural history. The question
is, "Would 10 years of tamoxifen be better than five years
for providing protection against recurrence in the second decade
after diagnosis?" There are almost as many breast cancer deaths
in the second decade after diagnosis as in the first. We've really
got to think on a long-term scale. When we do that, the idea that
10 years might be better than five years actually becomes quite
interesting. We've got virtually no information on that second decade.
The idea that there might be some serious adverse effect on breast
cancer from continuing tamoxifen beyond five years, has disappeared.
That, in retrospect, was just a chance fluctuation, a "zig"
in one trial, which has been counterbalanced by "zags"
elsewhere. Now it's just averaged out. So, I don't think there's
any reason to fear that longer treatment - for example, 10 years
of treatment - is going to be any worse in terms of having an adverse
affect on breast cancer itself. But whether it's going to actually
have any worthwhile benefit in that second decade, is still unanswered.
-Richard
Peto, FRS
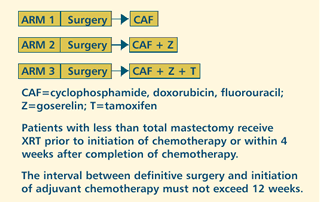
| PHASE
III RANDOMIZED COMPARISON OF ADJUVANT THERAPIES IN PREMENOPAUSAL
WOMEN WITH RESECTED NODE-POSITIVE HORMONE RECEPTOR-POSITIVE
ADENOCARCINOMA OF THE BREAST (Closed to accrual) Protocol
PROTOCOL
IDS: EST-5188, INT-0101
PROJECTED
ACCRUAL: 1,537 patients were entered into the trial.
|
 Davidson
NE. Hormonal ablation. 2000 NIH Consensus Development
Conference on Adjuvant Therapy for Breast Cancer; Abstract.
Davidson
NE. Hormonal ablation. 2000 NIH Consensus Development
Conference on Adjuvant Therapy for Breast Cancer; Abstract. |
INTERGROUP
0101 STUDY OF ADJUVANT OVARIAN ABLATION
The study
was designed a long time ago, and at a time when there was increasing
interest in ovarian ablation because of the meta-analysis and because
there were now drugs available that could lead to chemical castration
as opposed to surgical ablation.
In the best
of all worlds we would have had a 4th arm of CAF followed by tamoxifen,
but at the time it was felt that we weren't sure that we could pull
it off statistically - that we could accrue to that trial in a timely
fashion and have something to talk about.
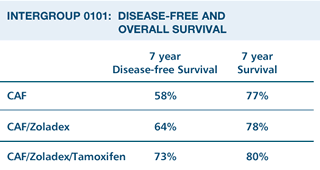
The disease-free
survival was better for the group receiving CAFZT compared to CAFZ.
There was a borderline impact of CAFZ compared to CAF. An unplanned
preliminary, retrospective subset analysis demonstrated that younger
women - arbitrarily defined as women under the age of 40 - seemed
to do better with Zoladex® (goserelin acetate implant). Perhaps
that's not surprising, because those women are the least likely
to be made postmenopausal by chemotherapy. There's also a suggestion
that women with premenopausal estrogen levels after chemotherapy
were destined to derive benefit from Zoladex.
The big clinical
question now is what to do with the young woman who is premenopausal
at the end of chemotherapy? Most of them receive tamoxifen as a
matter of routine, and I personally am not using LHRH agonists in
that situation right now, but some of our very good colleagues have
looked at these trial results and said that they think it is legitimate
to do.
-Nancy
E Davidson, MD
ADJUVANT
OVARIAN ABLATION IN A NONPROTOCOL SETTING
We now have
several trials demonstrating that in receptor-positive premenopausal
patients, ovarian ablation is as effective as CMF - and, in fact,
there's almost a suggestion that it is better. In the ECOG study
(Intergroup 0101), patients received CAF, which everyone would consider
state-of-the-art chemotherapy. In that trial, there was additional
benefit from adding ovarian ablation to CAF - certainly among women
under age 40. That study really changed my thinking, and I have
now moved to the point that if a woman receives adjuvant chemotherapy
and does not stop menstruating, I routinely add the LHRH agonist,
Zoladex with tamoxifen.
-I
Craig Henderson, MD
COMBINATION
ENDOCRINE THERAPY
Combination
endocrine therapy is a conceptual change for us. We eliminated that
approach a couple of decades ago, perhaps because of small, inadequately
powered trials that were clearly unable to detect the type of differences
we can identify today with larger studies. The reality is that combination
endocrine therapy may be more effective than single agent treatment.
With better tools now available, we are returning to the concept
of complete estrogen blockade - a strategy that started several
decades ago with hypophesectomy and adrenalectomy. Studies of chemical
ovarian suppression plus tamoxifen suggest greater efficacy than
either alone.
-Gabriel
Hortobagyi, MD
TAMOXIFEN
IN THE PREMENOPAUSAL PATIENT
Tamoxifen
seems to work much better in patients not actively menstruating.
So that's just another reason to make certain that the patient is
postmenopausal before you start adjuvant tamoxifen. I think that
a premenopausal woman who becomes postmenopausal and then receives
tamoxifen - assuming that she has an ER-positive tumor - is going
to get a lot more benefit. In fact, she should get the same benefit
as a postmenopausal woman. I've been using tamoxifen in premenopausal
women for quite a few years, but now I want to make sure they're
postmenopausal first.
-I
Craig Henderson, MD
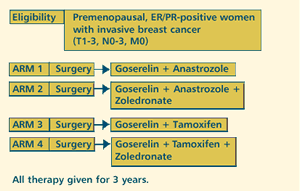
| OVARIAN
SUPPRESSION PLUS ANASTROZOLE OR TAMOXIFEN IN HORMONE RECEPTOR-POSITIVE,
NODE-NEGATIVE OR NODE-POSITIVE BREAST CANCER
PROTOCOL
ID: ABCSG-12
PROJECTED
ACCRUAL: 1,250 patients will be accrued to this study.
|
|
STUDY
CONTACT
Raimund
Jakesz, Chair Austrian Breast & Colon Cancer Study Group
Vienna, Austria
|
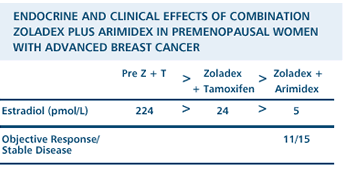
Derived
from Cheung KL et al. The combined use of goserelin and anastrozole
as second-line endocrine therapy in premenopausal women with advanced
breast cancer: A study of its clinical and endocrine effects.
Proc ASCO 2001; Abstract
1937.
ZOLADEX PLUS
ARIMIDEX®(ANASTROZOLE) AS ADJUVANT THERAPY
Endocrine
maneuvers are often limited in premenopausal patients, but we know
that premenopausal patients with ER-positive tumors are just as
likely to be sensitive to hormone therapy as postmenopausal women.
And so we decided that, for patients on tamoxifen and Zoladex, there
was no reason why we shouldn't treat in the same way as we would
have a postmenopausal woman who progresses on tamoxifen. You stop
the tamoxifen and start an aromatase inhibitor.
And so, we
had a series of women where that's what we did. We stopped the tamoxifen,
we continued the Zoladex, and then we substituted Arimidex for the
tamoxifen. And a significant percentage of those women responded.
-John
F Robertson, MD, FRCS
AUSTRIAN
TRIAL
There's a
trial that's now been started in Austria that has been looking at
Zoladex plus Arimidex as an adjuvant therapy. The key randomization
is to Zoladex plus tamoxifen or Zoladex plus Arimidex. The basis
for that is there was a study presented at ASCO whereby patients
were randomized either to CMF or to Zoladex plus tamoxifen.
This was
in premenopausal, ER-positive patients. And what it showed was that
the early recurrence-free survival curves were in favor of the endocrine
arm. And so they have taken that as the control arm for the next
study, and they're now comparing Zoladex plus tamoxifen to Zoladex
plus Arimidex.
-John
F Robertson, MD, FRCS
AROMATASE
INHIBITORS IN WOMEN MADE MENOPAUSAL BY LHRH AGONISTS
There is
one limited metastatic study in ER-positive patients demonstrating
that Zoladex plus anastrozole yielded similar responses to what
has been seen in postmenopausal patients, but we need to design
appropriate trials to address this issue.
It is very
important to consider that aromatase inhibitors as monotherapy should
not be used in premenopausal patients. But it would be a very interesting
and logical approach to design trials of complete estrogen blockade
in ER-positive premenopausal patients, using an LHRH agonist and
anastrozole.
-Aman
Buzdar, MD
| PHASE
III RANDOMIZED STUDY OF MEDROXYPROGESTERONE ACETATE VERSUS OBSERVATION
FOR PREVENTION OF ENDOMETRIAL PATHOLOGY IN PATIENTS WITH POSTMENOPAUSAL
BREAST CANCER TREATED WITH ADJUVANT TAMOXIFEN Protocol
PROTOCOL
IDS: SWOG-S9630; SWOG-9630
PROJECTED
ACCRUAL: A total of 208 patients (104 per arm) will be
accrued within 3 years.
|
|

OBJECTIVES
- Compare endometrial pathologic diagnoses for postmenopausal,
tamoxifen - treated breast carcinoma patients randomly assigned
to observation or cyclical medroxyprogesterone acetate (MA).
- Compare endometrial pathologic diagnoses resulting in
tamoxifen discontinuation and intermittent bleeding in breast
cancer patients receiving tamoxifen and randomized to observation
or cyclical MA.
- Characterize the incidence of spontaneous regression and
progression of simple or cystic hyperplasia.
- Characterize endometrial biopsy results using different
endometrial stripe width cut-off points for cases in which
the width is at least 5 mm by endovaginal ultrasound for
women receiving tamoxifen.
- Compare changes over time in endometrial oncogene expression
and receptor status for postmenopausal tamoxifen-treated
breast carcinoma patients with or without prior chemotherapy
randomly assigned to MA vs observation.
- Describe associations among change in gene expression,
receptor status, endometrial abnormality, length of tamoxifen
exposure and prior chemotherapy.
PARTICIPATION
CRITERIA
- Postmenopausal women aged 18 and over
- Candidate for adjuvant tamoxifen (Patients must star t
tamoxifen or have started within 28 days prior to study
and plan to continue for 5 years)
- Histologically proven primary invasive stage I, IIA or
IIB breast cancer (T1-3, N0-1, M0)
- Prior definitive local treatment of primary lesion
- Patients with BCT must have received or be planning to
receive XRT at the start of tamoxifen treatment
- No endometrial hyperplasia, proliferative changes, complex
or atypical hyperplasia or carcinoma
STUDY
CONTACT
Ronald
Keith Potkul, Chair, Ph: 708-327-3314 Southwest Oncology Group
Seema Khan, Chair, Ph: 315-464-6276 Cancer and Leukemia Group
B
|
| STUDY
OF TAMOXIFEN IN THE PREVENTION OF SKELETAL AND CARDIOVASCULAR
MORBIDITY OF ADJUVANT CHEMOTHERAPY IN PREMENOPAUSAL WOMEN WITH
STAGE I OR II BREAST CANCER Protocol
PROTOCOL
IDS: NU-95B2; NCI-G00-1737
PROJECTED
ACCRUAL: A total of 80 patients (40 per stratum) will
be accrued.
|
OBJECTIVES
- Compare the bone density in the femoral neck and lumbar
spine and cholesterol levels in premenopausal women with
stage I or II breast cancer treated with adjuvant chemotherapy
with or without tamoxifen.
- Collect information regarding breast cancer risk factors,
treatment, pathology, diet, activity, weight and smoking.
PARTICIPATION
CRITERIA
- Premenopausal women ages 30-50
- Histologically proven stage I or II breast cancer
- Scheduled to receive adjuvant chemotherapy +/- tamoxifen
PROTOCOL
Adjuvant
chemotherapy +/- tamoxifen at the discretion of the treating
physician. Baseline levels of FSH, estradiol, total cholesterol,
HDL, LDL measured. Baseline bone densitometry of the femoral
neck and lumbar spine. Laboratory studies and bone densitometry
repeated at years 1 and 2.
STUDY
CONTACT
Monica
Morrow, Chair, Ph: 312-926-9039 Robert H Lurie Comprehensive
Cancer Center Northwestern University, Chicago, Illinois
|
| PHASE
III RANDOMIZED STUDY OF ADJUVANT TAMOXIFEN, OVARIAN SUPPRESSION,
AND/OR CHEMOTHERAPY IN WOMEN WITH STAGE I, II AND IIIA BREAST
CANCER Protocol
PROTOCOL
IDS: UKCCCR-ABC; EU-94029
PROJECTED
ACCRUAL: Approximately 6,000 women (4,000 premenopausal,
2,000 postmenopausal) will be accrued.
|
|
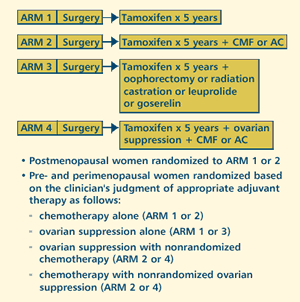
OBJECTIVES
- Estimate overall and relapse-free survival of women with
early-stage breast cancer receiving adjuvant tamoxifen with
or without adjuvant chemotherapy and/or ovarian suppression.
PARTICIPATION
CRITERIA
- Pre-, peri- or postmenopausal
- Hormone receptor status: Not specified
- Histologically confirmed invasive breast cancer (stage
I, II or IIIA) for which adjuvant therapy is appropriate
- Pathologically positive or negative nodes
- Any tumor size
STUDY
CONTACT
John Robert
Yarnold, Chair, Ph: 020-8661-3891 United Kingdom Coordinating
Committee on Cancer Research-ABC
|
| PHASE
III RANDOMIZED STUDY OF ADJUVANT TAMOXIFEN WITH OR WITHOUT OVARIAN
SUPPRESSION AND/OR CYCLOPHOSPHAMIDE, METHOTREXATE AND FLUOROURACIL
(CMF) IN PREMENOPAUSAL WOMEN WITH STAGE I-IIIA, UNILATERAL,
INVASIVE BREAST CANCER Protocol
PROTOCOL
IDS: SCTN-BR9401; EU-94002; UKCCCR-ABC/BR9401
PROJECTED
ACCRUAL: : A total of 1,000 patients will be accrued.
|
|
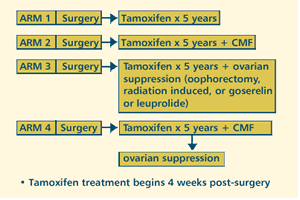
OBJECTIVES
- Compare the potential benefits of adjuvant tamoxifen with
or without ovarian suppression and/or CMF in premenopausal
women with stage I-IIIA unilateral, invasive breast cancer.
PARTICIPATION
CRITERIA
- Age: 70 and under
- Premenopausal
- Hormone receptor status: Not specified
- Histologically proven unilateral, invasive breast cancer
(T0-3, N0-1, M0)
STUDY
CONTACT
WD George,
Chair, Ph: 0141-211-2166 Scottish Cancer Therapy Network
|
| PHASE
III RANDOMIZED STUDY OF ADJUVANT TAMOXIFEN WITH OR WITHOUT CYCLOPHOSPHAMIDE,
METHOTREXATE AND FLUOROURACIL (CMF) IN POSTMENOPAUSAL WOMEN
WITH STAGE I-IIIA, UNILATERAL, INVASIVE BREAST CANCER Protocol
PROTOCOL
IDS: : SCTN-BR9402; EU-94003; UKCCCR-ABC/BR9402
PROJECTED
ACCRUAL: A total of 1,000 patients will be accrued.
|
|
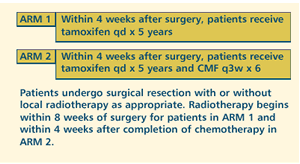
OBJECTIVES
- Compare the potential benefits of adjuvant tamoxifen with
or without cyclophosphamide, methotrexate and fluorouracil
(CMF) in postmenopausal women with stage I-IIIA, unilateral,
invasive breast cancer.
PARTICIPATION
CRITERIA
- Age: 70 and under
- Postmenopausal
- Hormone receptor status: Not specified
- Histologically proven unilateral, invasive breast cancer
(stage T0-3, N0-1, M0)
- No evidence of distant disease, including ipsilateral
supraclavicular node enlargement unless proven benign
- No carcinoma in situ alone, including Paget's disease
of the nipple without underlying invasion
- No history of pure carcinoma in situ of either breast
STUDY
CONTACT
WD George,
Chair, Ph: 0141-211-2166 Scottish Cancer Therapy Network
|
| PHASE
III RANDOMIZED STUDY OF SURGERY WITH OR WITHOUT AXILLARY NODE
CLEARANCE FOLLOWED BY ADJUVANT TAMOXIFEN IN ELDERLY WOMEN WITH
BREAST CANCER Protocol
PROTOCOL
IDS: IBCSG-10-93; NCI-F93-0008; EU-93013
PROJECTED
ACCRUAL: A total of 1,020 patients will be accrued in
approximately 5 years.
|
|
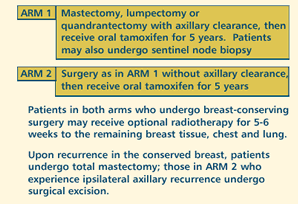
OBJECTIVES
- Determine local and systemic disease-free survival, ipsilateral
axillary relapse, occurrence of postmastectomy syndrome,
overall survival and toxicity of breast surgery with versus
without axillary node dissection in elderly women with clinically
operable stage I or IIA breast cancer who subsequently receive
adjuvant tamoxifen.
- Compare the quality of life in patients treated with the
two regimens.
PARTICIPATION
CRITERIA
- Age: 60 and over
- Postmenopausal
- Hormone receptor status: Not specified
- Histologically or cytologically diagnosed stage I or IIA
breast carcinoma that is potentially operable
- No prior axillary clearance or biopsy
- Complete excisional biopsy without axillary clearance
or biopsy allowed
- Suspicious manifestations of metastatic disease must be
proven benign
- No bilateral breast cancer
STUDY
CONTACT
Diana
Crivellari, Chair, Ph: 39-434-659206 International Breast
Cancer Study Group
|
Page
2 of 2
Previous | Select Publications
Top
Additional
Sections:
1 | 2
| 3 | 4
| 5 | 6
| 7 | 8
| 9 | 10
| 11 | 12
|
|
|

