|
 Home:
Educational
Supplement: Section 3
Home:
Educational
Supplement: Section 3
RADIATION
THERAPY OVERVIEW
The most
recent overview involved the examination of the deaths of more than
10,000 women out of a total of about 20,000 women in 40 randomized
trials worldwide. No clear effect of radiotherapy on total mortality
was found, but the study found highly significant differences in
breast cancer deaths and nonbreast-cancer deaths. Both of these
changes were highly significant. The change in non-breast-cancer
deaths emerged later than the change in breast cancer deaths, the
differences being 1.0 percent for breast cancer mortality and 3.0
percent for non-breast-cancer mortality at 10 years. Most of the
excess non-breast-cancer deaths were due to vascular disease, which
increased by 30 percent.
-Jack
Cuzick, PhD
2000 NIH Consensus Conference. Abstract
POSTMASTECTOMY
RADIATION THERAPY
There is
evidence that women with a high risk of locoregional tumor recurrence
after mastectomy will benefit from postoperative radiotherapy. This
high-risk group includes women with four or more positive lymph
nodes or an advanced primary tumor. ... At this time, the role of
postmastectomy radiotherapy for women with one to three positive
lymph nodes remains uncertain and is being examined in a randomized
clinical trial.
-2000
NIH Consensus Statement. Full-Text
INDIVIDUALIZING
POSTMASTECTOMY RADIATION THERAPY
This is a
very interesting question that challenges the concept we've had
for so many years that breast cancer is a systemic disease. While
one can quibble with aspects of the Danish and British Columbia
trials, it is important that the subset that seemed to benefit the
most in terms of survival - women with small tumors with a limited
number of positive nodes - is consistent with everything else we
believe about aggressive local-regional therapy.
The ongoing
Intergroup trial is very important. This study is using modern radiotherapy
techniques, and one would hope that the incidence of late cardiac
morbidity is going to be very low. In a nonprotocol setting, we
evaluate these one to three node-positive cases individually and
discuss radiation therapy in patients with large nodal metastases,
extracapsulary extension and large primary tumors, particularly
with lots of lymphatic invasion in the breast. We also discuss this
option in a woman who is very anxious to minimize her risk of failure
and wants to opt for treatments that may give very little benefit.
Node-positive disease is a continuum. I suspect that this will also
be true of the benefits of postmastectomy radiation therapy.
-Monica
Morrow, MD
| PHASE
III RANDOMIZED STUDY OF TAMOXIFEN WITH OR WITHOUT RADIOTHERAPY
IN WOMEN WITH DUCTAL CARCINOMA IN SITU (DCIS) OF THE BREAST
Protocol
PROTOCOL
IDS: RTOG-9804, RTOG-DEV-1026, CTSU
PROJECTED
ACCRUAL: A total of 1,990 patients will be accrued for
this study over 6 years.
|
|

OBJECTIVES
- Compare the efficacy of tamoxifen with or without whole
breast radiation in decreasing or delaying the appearance
of local failure, both invasive and in situ, and preventing
the need for mastectomy in women with ductal carcinoma in
situ (DCIS) of the breast.
- Compare distant disease-free survival of these patients
in these treatment arms.
PARTICIPATION
CRITERIA
- Age: 26 and over
- Primary tumor no greater than 5 cm
- Ductal carcinoma in situ of the breast detected by mammogram
at the time of diagnosis
- Unicentric
- Lesions no greater than 2.5 cm
- Low or intermediate grade
- Inked margins at least 3 mm
- Clinically node-negative
STUDY
CONTACT
Beryl
McCormick, Chair, Ph: 212-639-6828
Radiation Therapy Oncology Group
Barbara
L Smith, Chair, Ph: 617-724-4800
Cancer and Leukemia Group B
|
| PHASE
II RANDOMIZED STUDY OF VITAMIN E AND PENTOXIFYLLINE IN WOMEN
WITH LYMPHEDEMA AFTER RADIOTHERAPY FOR BREAST CANCER Protocol
PROTOCOL
IDS: RM-1597, EU-20050
PROJECTED
ACCRUAL: A total of 100 patients (50 per treatment arm)
will be accrued for this study.
|
|

OBJECTIVES
- Determine the effects of vitamin E and pentoxifylline
on lymphedema in women previously treated with radiotherapy
for breast cancer.
- Assess the normal tissue injury and quality of life in
patients treated with this regimen compared to placebo.
PARTICIPATION
CRITERIA
- Prior diagnosis of breast cancer (TI-3, NO-1, MO)
- Prior radiotherapy to breast/chest wall plus axilla and/or
stem cell transplantation
- At least five years since prior radiotherapy
- No disease recurrence
- Arm lymphedema due to prior radiotherapy
- Reduced shoulder movement, induration in breast/chest
wall, radiation-induced brachial plexopathy, symptomatic
lung fibrosis or nonhealing wounds (including fractures)
allowed as evidence of disability in addition to arm lymphedema
- No prior axillary surgery
- Lower axillary sampling allowed
- At least three months since prior daily vitamin E supplementation
more than 30 mg/day
- No prior pentoxifylline after radiotherapy
- No concurrent ketorolac or vitamin K
- No other concurrent vitamin E supplementation
STUDY
CONTACT
John Robert
Yarnold, Chair, Ph: 020-8661-3891
Royal Marsden Hospital
Sutton, England, United Kingdom
|
| PHASE
III RANDOMIZED STUDY OF SYNCHRONOUS VERSUS SEQUENTIAL ADJUVANT
CHEMOTHERAPY AND RADIOTHERAPY IN WOMEN WITH EARLY STAGE BREAST
CANCER Protocol
PROTOCOL
IDS: CRC-TU-BR3015, EU-99005
PROJECTED
ACCRUAL: A total of 2,000 patients (1,000 per treatment
arm) will be accrued for this study.
|
|
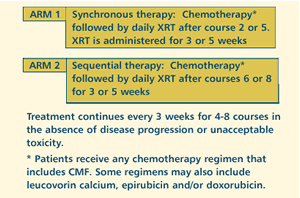
OBJECTIVES
- Compare the effects of synchronous versus sequential adjuvant
chemotherapy and radiotherapy on local recurrence, disease-free
and overall survival, and treatment delay in women with
early stage breast cancer.
- Compare the safety with regard to dose intensity and toxicity
of these treatment regimens. Evaluate the quality of life
and cosmetic outcome.
- Evaluate the quality of life and cosmetic outcome.
PARTICIPATION
CRITERIA
- Histologically confirmed early stage, invasive, unilateral
breast cancer
- Planned use of adjuvant chemotherapy and radiotherapy
- Prior or concurrent hormonal therapy allowed
STUDY
CONTACT
Indy Fernando,
Ph: 0121-414-3787
Cancer Research Campaign Trials Unit
University of Birmingham
Birmingham, England, United Kingdom
|
| PHASE
II STUDY OF CONCURRENT PACLITAXEL AND RADIOTHERAPY FOLLOWING
ADJUVANT DOXORUBICIN AND CYCLOPHOSPHAMIDE IN WOMEN WITH STAGE
II OR III BREAST CANCER Protocol
PROTOCOL
IDS: CWRU-2199, NCI-G00-1851, BMS-CWRU-2199
PROJECTED
ACCRUAL: Approximately 40 patients will be accrued for
this study over 12-18 months.
|
OBJECTIVES
- Determine the feasibility of concurrent paclitaxel and
breast radiotherapy in women with stage II or III breast
cancer who have had primary breast-conserving surgery and
adjuvant chemotherapy.
- Assess the cosmetic results of breast conservation after
this treatment.
- Determine the pulmonary toxicity of this regimen.
PARTICIPATION
CRITERIA
- Stage II or III invasive breast cancer
- Prior breast-conserving surgery with axillary lymph node
dissection required
- Adjuvant AC completed within past 3 weeks
- Prior tamoxifen allowed, no concurrent tamoxifen
PROTOCOL
Paclitaxel
IV x 4 three weeks after last AC adjuvant regimen. Concurrent
XRT x approximately 6-7 weeks. Treatment continues in the
absence of disease progression or unacceptable toxicity.
STUDY
CONTACT
Beth A.
Overmoyer, Chair, Ph: 216-844-5176
Ireland Cancer Center
Cleveland, Ohio
|
| PHASE
III RANDOMIZED STUDY OF RADIOTHERAPY FRACTIONATION REGIMENS
AFTER LOCAL EXCISION OR MASTECTOMY IN WOMEN WITH EARLY STAGE
BREAST CANCER Protocol
PROTOCOL
IDS: EU-99014, STMG-STARTA
PROJECTED
ACCRUAL: A total of 2,010 patients (670 per arm) will
be accrued for this study.
|
|
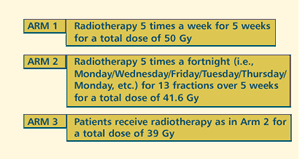
OBJECTIVES
- Determine the benefits of radiotherapy schedules using
fraction sizes larger than 2.0 Gy in terms of normal tissue
responses, local-regional tumor control, quality of life
and economic consequences in women prescribed postoperative
radiotherapy for early stage breast cancer.
PARTICIPATION
CRITERIA
- Histologically confirmed invasive unilateral breast cancer
that is considered operable. T1-3, N0-1, M0 at presentation
- Complete macroscopic excision of tumor by breast-conserving
surgery or mastectomy
- No immediate breast reconstruction
- No requirement for axillary radiotherapy after greater
than a Level 1 axillary dissection or after greater than
10 lymph nodes have been removed
- Prior neoadjuvant, or primary medical, therapy allowed
provided subsequent surgery confirms complete macroscopic
excision of residual primary tumor
- At least 2 weeks since prior cytotoxic agents
- No concurrent chemotherapy
STUDY
CONTACT
John Robert
Yarnold, Ph: 020-8661-3891
START Trial Management Group
Royal Marsden Hospital
Sutton, England, United Kingdom
|
| PHASE
III RANDOMIZED STUDY OF INTERNAL MAMMARY AND MEDIAL SUPRACLAVICULAR
LYMPH NODE CHAIN IRRADIATION VS NO FURTHER THERAPY IN WOMEN
WITH RESECTED STAGE I/II/III BREAST CANCER Protocol
PROTOCOL
IDS: EORTC-10925
PROJECTED
ACCRUAL: A total of 4,000 patients will be accrued for
this study within 4 years.
|
|
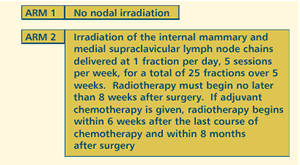
OBJECTIVES
- Compare the effect of irradiation of the homolateral internal
mammary and medial supraclavicular lymph node chains vs
no further therapy on survival, disease-free survival, metastasis-free
survival and cause of death in women with resected stage
I/II/III breast cancer.
PARTICIPATION
CRITERIA
- Histologically confirmed unilateral adenocarcinoma of
the breast
- Stage I/II/III (Tx, T0-3, N0-2) disease in one of the
following categories or multifocal tumors if one of the
foci is in agreement with the following:
- Centrally or medially located with any lymph node status
- Central location defined as underlying the areola
- Medial location defined as at least partial involvement
of upper or lower medial quadrant of breast
- Externally located with axillary node involvement
- Prior mastectomy or breast-conserving surgery and axillary
dissection required
- Sentinel node procedure as axillary intervention without
further axillary surgery is allowed
- No prior internal mammary chain dissection
- No upper inner lesion treated with breast-conserving
surgery that precludes sparing of internal mammary lymph
node chain from radiotherapy volume
- Decision at radiation oncologist's discretion
STUDY
CONTACT
Walter
F. Van den Bogaert, Ph: 32-16346902
EORTC Breast Cancer Group
U.Z. Gasthuisberg
Leuven, Belgium
|
| PHASE
III RANDOMIZED STUDY OF ADJUVANT BREAST RADIOTHERAPY WITH OR
WITHOUT REGIONAL RADIOTHERAPY IN WOMEN WITH RESECTED, EARLY
STAGE, INVASIVE BREAST CANCER Protocol
PROTOCOL
IDS: CAN-NCIC-MA20
PROJECTED
ACCRUAL: Approximately 1,822 patients will be accrued
for this study within approximately 4 years.
|
|

OBJECTIVES
- Compare the overall survival, disease-free survival, isolated
local regional disease-free survival and distant disease-free
survival in women with resected, early stage, invasive breast
cancer treated with breast radiotherapy with or without
regional radiotherapy.
- Compare the toxicities, including cosmetic outcomes, of
these 2 regimens in these patients.
- Compare the quality of life of patients treated with these
2 regimens.
PARTICIPATION
CRITERIA
- Histologically proven invasive carcinoma of the breast
without evidence of T4, N2-3, or M1 disease prior to surgery
- Node-positive or high-risk node-negative
- Prior breast-conserving therapy (BCT) (e.g., lumpectomy,
partial mastectomy, or segmental mastectomy) and axillary
node dissection or sentinel node biopsy required and must
be a candidate for breast radiotherapy after BCT
- Patients with positive margins should undergo re-excision
- Patients with microscopically focally positive margins
defined as no greater than 3 times high power fields are
candidates for breast radiotherapy plus a boost to the lumpectomy
site
- Treated with currently accepted adjuvant systemic chemotherapy
and/or hormonal therapy
- High risk of regional and systemic recurrence due to one
of the following:
- Pathologically positive axillary lymph nodes
- Pathologically negative axillary lymph nodes with one
of the following:
- Primary tumor greater than 5 cm
- Primary tumor greater than 2 cm and less than 10 axillary
lymph nodes excised and one of the following:
- Estrogen receptor-negative or Skarf-Bloom - Richardson
grade 3 or lymphovascular invasion
- Estrogen and progesterone receptor status known
STUDY
CONTACT
Timothy
Joseph Whelan, Ph: 905-387-9711 ext. 64501
NCIC-Clinical Trials Group
Cancer Care Ontario-Hamilton Regional Cancer Centre
Hamilton, Ontario, Canada
|
| PHASE
III RANDOMIZED STUDY OF RADIOTHERAPY FRACTIONATION REGIMENS
AFTER LOCAL EXCISION OR MASTECTOMY IN WOMEN WITH EARLY STAGE
BREAST CANCER Protocol
PROTOCOL
IDS: STMG-STARTB, EU-99015
PROJECTED
ACCRUAL: A total of 1840 patients (920 per arm).
|
|

OBJECTIVES
- Determine the benefits of radiotherapy schedules using
fraction sizes larger than 2.0 Gy in terms of normal tissue
responses, local-regional tumor control, quality of life
and economic consequences in women prescribed postoperative
radiotherapy for early stage breast cancer
PARTICIPATION
CRITERIA
- Histologically confirmed invasive unilateral breast cancer,
T1-3, N0-1, M0 at presentation
- Complete macroscopic excision of tumor by breast-conserving
surgery or mastectomy
- No immediate breast reconstruction
- No requirement for axillary radiotherapy after greater
than a Level 1 axillary dissection or after greater than
10 lymph nodes have been removed
- At least 2 weeks since prior cytotoxic agents
- No concurrent chemotherapy
STUDY
CONTACT
John Robert
Yarnold, Ph: 020-8661-3891
START Trial Management Group
Royal Marsden Hospital, Sutton, England, United Kingdom
|
| PHASE
III RANDOMIZED STUDY OF COMPLETE AXILLARY LYMPH NODE DISSECTION
VERSUS AXILLARY RADIOTHERAPY IN SENTINEL LYMPH NODE-POSITIVE
WOMEN WITH OPERABLE INVASIVE BREAST CANCER Protocol
PROTOCOL
IDS: EORTC-10981, EORTC-10981-AMAROS
PROJECTED
ACCRUAL: A total of 3,485 patients will be accrued for
this study within three years.
|
|

OBJECTIVES
- Compare the overall survival, disease-free survival, isolated
local regional disease-free survival and distant disease-free
survival in women with resected, early stage, invasive breast
cancer treated with breast radiotherapy with or without
regional radiotherapy.
- Compare the toxicities, including cosmetic outcomes, of
these 2 regimens in these patients.
- Compare the quality of life of patients treated with these
2 regimens.
PARTICIPATION
CRITERIA
- Histologically proven invasive carcinoma of the breast
without evidence of T4, N2-3, or M1 disease prior to surgery
- Node-positive or high-risk node-negative
- Prior breast-conserving therapy (BCT) (e.g., lumpectomy,
partial mastectomy, or segmental mastectomy) and axillary
node dissection or sentinel node biopsy required and must
be a candidate for breast radiotherapy after BCT
- Patients with positive margins should undergo re-excision
- Patients with microscopically focally positive margins
defined as no greater than 3 times high power fields are
candidates for breast radiotherapy plus a boost to the lumpectomy
site
- Treated with currently accepted adjuvant systemic chemotherapy
and/or hormonal therapy
- High risk of regional and systemic recurrence due to one
of the following:
- Pathologically positive axillary lymph nodes
- Pathologically negative axillary lymph nodes with one
of the following:
- Primary tumor greater than 5 cm
- Primary tumor greater than 2 cm and less than 10 axillary
lymph nodes excised and one of the following:
- Estrogen receptor - negative or Skarf - Bloom -
Richardson grade 3 or lymphovascular invasion
- Estrogen and progesterone receptor status known
STUDY
CONTACT
Timothy
Joseph Whelan, Ph: 905-387-9711 ext. 64501
NCIC-Clinical Trials Group
Cancer Care Ontario-Hamilton Regional Cancer Centre
Hamilton, Ontario, Canada
|
PHASE
III RANDOMIZED STUDY OF WIDE LOCAL EXCISION ALONE VERSUS WIDE
LOCAL EXCISION FOLLOWED BY RADIOTHERAPY WITH OR WITHOUT TAMOXIFEN
VERSUS WIDE LOCAL EXCISION PLUS TAMOXIFEN IN PATIENTS
WITH STAGE I BREAST CANCER Protocol
PROTOCOL
IDS: BASO-BREAST-BASO-II, EU-20019
PROJECTED
ACCRUAL: A total of 1,200 patients (300 per treatment
arm).
|
|

OBJECTIVES
- Compare the effectiveness of wide local excision alone
versus wide local excision followed by radiotherapy with
or without tamoxifen versus wide local excision plus tamoxifen
in patients with stage I breast cancer.
- Assess local occurrence in the treated breast, regional
recurrence, distant recurrence, death from breast cancer
and occurrence of cancer in the opposite breast in these
patients when treated with one of these regimens.
PARTICIPATION
CRITERIA
- Age: Under 70
- Histologically confirmed unilateral stage I invasive breast
cancer
- No more than 1 tumor and no greater than 2 cm
- No vascular invasion
- No other concurrent adjuvant systemic therapy
STUDY
CONTACT
R W Blamey,
Ph: 115 969 1169
British Association of Surgical Oncology: Breast Group
Nottingham City Hospital NHS Trust
Nottingham, England, United Kingdom
|
| PHASE
II STUDY OF ADJUVANT RADIATION THERAPY AFTER RESECTION OF BORDERLINE
AND MALIGNANTPHYLLODES TUMORS Protocol
PROTOCOL
IDS: DMS-9801,
NCI-V98-1442
PROJECTED
ACCRUAL: A
total of 20 patients will be accrued for this study over 3-4
years.
|
|
OBJECTIVES
- Determine the local recurrence rate for patients with
phyllodes tumors of the breast treated with local excision
with negative margins and adjuvant radiation therapy.
- Determine the survival rate in this patient population.
PARTICIPATION
CRITERIA
- Histologically proven phyllodes tumors of the breast with
borderline or malignant grade
- Borderline 5 to 9 mitoses/10 high power fields (HPF),
pushing or infiltrating margins, 2+ atypia
- Malignant: 10 or more mitoses/10 HPF, predominantly
infiltrating margins, usually 3+ atypia with occasional
2+ atypia
- Must have been excised with breast-conserving resection
- No positive margins
- Local recurrence of a previously excised phyllodes tumor
allowed if the recurrence is in the area of the previous
excision
- No prior breast carcinoma or in situ ductal carcinoma
in the ipsilateral breast
PROTOCOL
Radiation
therapy five days per week for a total of 28 treatments. The
adjuvant radiation therapy must begin within 12 weeks of local
excision or breast re-excision.
STUDY
CONTACT
Richard
J. Barth, Jr., Ph: 603-650-7903
Norris Cotton Cancer Center
Lebanon, New Hampshire
|
| PHASE
III RANDOMIZED STUDY OF PALLIATIVE RADIATION THERAPY FOR BONE
METASTASES FROM BREAST OR PROSTATE CANCER Protocol
PROTOCOL IDS: RTOG-9714,
NCI-P97-0124
PROJECTED
ACCRUAL: This
study will accrue 938 patients within 2 years.
|
|

OBJECTIVES
- Compare the ability of a single fraction of radiation
therapy vs multiple fractions to provide complete pain relief
in patients with painful bone metastases from breast or
prostate cancer.
- Determine the frequency and duration of pain relief and
narcotic relief after these treatments in these patient
populations.
- Compare the effect on quality of life of these two treatments
in these patient populations.
- Compare the incidence of pathologic fracture within the
treatment fields after these two treatments in these patient
populations.
PARTICIPATION
CRITERIA
- Histologically proven breast or prostate cancer
- Radiographic evidence of bone metastasis within 8 weeks
of study
- Eligible treatment sites:
- Weight-bearing sites: pelvis (excluding pubis), femur,
sacrum and/or sacroiliac joints, tibia
- Nonweight-bearing sites: up to 5 consecutive cervical,
thoracic or lumbar vertebral bodies, lumbosacral spine,
up to 3 consecutive ribs, humerus, fibula, radius with/without
ulna, clavicle, scapula, pubis
- If multiple sites are treated, site is included as weight-bearing
if any of the sites include the pelvis, sacrum, femur or
tibia
- Worst pain score of at least 5 on a scale of 10
- No skull, feet or hand metastases
- No spinal cord or cauda equina compression/effacement
in vertebral metastases
- Multiple sites eligible if they can be included in no
greater than 3 treatment sites
- No planned surgical fixation of the bone
STUDY
CONTACT
William
F Hartsell, Ph: 847-723-8030
Radiation Therapy Oncology Group
Lutheran General Cancer Care Center
Park Ridge, Illinois
Ivy A
Peterson, Chair, Ph: 507-284-2669
North Central Cancer Treatment Group
|
Page
2 of 2
Back | Select Publications
Top
Additional
Sections:
1 | 2
| 3 | 4
| 5 | 6
| 7 | 8
| 9 | 10
| 11 | 12
|
|
|

