|
 Home:
Educational
Supplement: Section 2
Home:
Educational
Supplement: Section 2
COMMENTS
FROM SURGICAL RESEARCH LEADERS ON SENTINEL NODE TRIALS
There are
three reasons to do axillary dissection: regional control, staging
and to improve survival. For staging, we have got enough literature
from around the world to tell us the accuracy of sentinel node biopsy.
For regional control, surgery results in almost 100% control, as
does radiation therapy, so before we abandon something that works
very well, we have to be very careful. We don't have any long-term
data on regional control for sentinel node. Regarding the issue
of survival - and I know it is a little heretical to say this -
there may be a survival advantage in controlling the axilla. The
few studies that looked at this were done in an era when we randomized
hundreds of patients, not thousands of patients. So the statistical
power was not there.
I've personally
never done a sentinel node procedure in a breast cancer case outside
of a clinical trial. I'm not going to say that it shouldn't be done
- this is a judgment call. But in terms of making the claim that
sentinel node is as good as axillary dissection, we don't have the
data and we are in an era of evidence-based medicine.
-David
Krag, MD
Many surgeons
believe that axillary dissection is therapeutic, and they are reluctant
not to perform axillary dissection in sentinel node-positive patients.
However, a number of randomized studies have failed to show that
axillary dissection improves survival. In sentinel node-positive
women, the sentinel node may be enough because often it's the only
involved node.
In addition,
virtually all node-positive women in this country receive adjuvant
systemic therapy, which may take care of any residual problem in
the axilla. Many patients are also receiving opposed tangential
field radiation, and that's partial axillary radiation.
In studies
where patients received lumpectomy with radiation and no axillary
dissection, the axillary recurrence rate was extraordinarily low.
I think ACOS Z-11 is a very important, very justifiable and ethical
trial. For an operation that's been used for 100 years, it's time
to answer the question about the need for axillary dissection.
-Armando
Giuliano, MD
|
A PHASE
III PROGNOSTIC STUDY OF SENTINEL NODE AND BONE MARROW MICROMETASTASES
IN WOMEN WITH STAGE I OR IIA BREAST CANCER Protocol
PROTOCOL
IDS: ACOSOG-Z0010, GUMC-00152
PROJECTED
ACCRUAL: Approximately 7,600 patients will be accrued
for this study within 3.8 years.
|
|
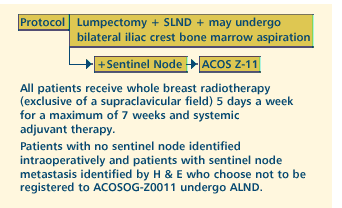
OBJECTIVES
- Estimate the prevalence and evaluate the prognostic significance
of sentinel lymph node micrometastases detected by immunohistochemistry
in women with stage I or IIA breast cancer.
- Estimate the prevalence and evaluate the prognostic significance
of bone marrow micrometastases detected by immunocytochemistry
for the first 3,600 women in this patient population.
- Evaluate the hazard rate for regional recurrence in women
whose sentinel nodes are negative by hematoxylin and eosin
(H&E) staining.
- Provide a mechanism for identifying women whose sentinel
nodes contain metastases detected by H&E, so that these
women can be considered as candidates for ACOSOG-Z0011.
PARTICIPATION
CRITERIA
- Histologically or cytologically confirmed stage I or IIA
(T1 or T2 N0 M0) breast carcinoma diagnosed within 60 days
of planned sentinel lymph node dissection
- Tumor must not be attached to the skin, underlying muscle
or chest wall
- No contralateral, supraclavicular or infraclavicular node
involvement
- No bilateral breast malignancies and no more than 1 malignant
tumor mass present in the same breast
- Tumor must be amenable to segmental mastectomy (lumpectomy)
- Medial quadrant lesions must have axillary drainage demonstrated
- Surgery: No prior ipsilateral axillary surgery, excisional
biopsy cavity exceeding 6 cm in diameter or prepectoral
breast implant
|
| A
PHASE III RANDOMIZED STUDY OF AXILLARY LYMPH NODE DISSECTION
IN WOMEN WITH STAGE I OR IIA BREAST CANCER WHO HAVE A POSITIVE
SENTINEL NODE Protocol
PROTOCOL
IDS: ACOSOG-Z0011, GUMC-00153
PROJECTED
ACCRUAL:
Approximately 1,900 patients will be accrued for this study
over 3.8 years.
|
|
OBJECTIVES
- Determine whether axillary lymph node dissection (ALND)
improves overall survival in patients with stage I or IIA
breast cancer.
- Quantify and compare surgical morbidities associated with
sentinel lymph node dissection with or without ALND in these
patients.
PARTICIPATION
CRITERIA
- No matted lymph nodes or gross extranodal disease
- Sentinel node must have been identified and found to contain
metastatic disease
- Histologically or cytologically confirmed stage I or IIA
breast carcinoma amenable to lumpectomy
- Tumor must not have been attached to the skin, underlying
muscle or chest wall
- No bilateral breast malignancies, and no more than 1 malignant
tumor mass present in the same breast
- No more than 2 positive lymph nodes
- Lumpectomy margins must be free of disease
- Surgery: No prepectoral implant; no prior ipsilateral
axillary surgery
STUDY
CONTACT
Armando
E Giuliano, Ph: 310-829-8089
John Wayne Cancer Institute
Santa Monica, California
|
| PHASE
III RANDOMIZED STUDY OF QUADRANTECTOMY WITH OR WITHOUT AXILLARY
LYMPH NODE DISSECTION FOLLOWED BY TAMOXIFEN IN WOMEN WITH STAGE
I, INVASIVE BREAST CANCER Protocol
PROTOCOL
IDS: CNR-9502, EU-95020
PROJECTED
ACCRUAL: 642 patients will be accrued over 3 years.
|
|

OBJECTIVES
- Assess the efficacy of quadrantectomy vs quadrantectomy
with axillary lymph node dissection followed by tamoxifen
in patients with stage I breast cancer, with efficacy measured
by local and distant relapse rates and by overall survival.
- Study the relationship between biological variables, such
as hormone receptor status, cell proliferation and DNA ploidy,
and the clinical outcome of the disease.
PARTICIPATION
CRITERIA
- Age range: 65 to 80
- Postmenopausal
- Histologically confirmed invasive breast cancer
- Clinical stage I (T1, N0, M0)
- Hormone receptor status:
- Estrogen receptor-positive
- Progesterone receptor-positive or -negative
STUDY
CONTACT
Gabriele
Martelli, Ph: 39-2-2390-324
Chemo Prevention Unit
Istituto Nazionale per lo Studio e la Cura dei Tumori
Milano (Milan), Italy
|
| PHASE III RANDOMIZED STUDY OF SURGERY
WITH OR WITHOUT AXILLARY NODE CLEARANCE FOLLOWED BY ADJUVANT
TAMOXIFEN IN ELDERLY WOMEN WITH BREAST CANCER Protocol
PROTOCOL IDS: IBCSG-10-93, NCI-F93-0008, EU-93013
PROJECTED ACCRUAL: A total of 1,020 patients will
be accrued for this study over approximately 5 years.
|
|
OBJECTIVES
- Determine
local and systemic disease-free survival, ipsilateral axillary
relapse, occurrence of postmastectomy syndrome, overall
survival and toxicity of breast surgery with vs without
axillary node dissection in elderly women with clinically
operable stage I or IIA breast cancer who subsequently receive
adjuvant tamoxifen.
- Compare
the quality of life in patients treated with the two regimens.
PARTICIPATION
CRITERIA
Age: 60
and over
Postmenopausal
Histologically or cytologically diagnosed stage I or IIA breast
carcinoma that is considered operable
No prior axillary clearance or biopsy allowed
Complete excisional biopsy without axillary clearance or biopsy
allowed
STUDY
CONTACT
Diana
Crivellari, Chair, Ph: 39-434-659206
International Breast Cancer Study Group
Centro di Riferimento Oncologico - Aviano
Aviano, Italy
|
| RANDOMIZED
STUDY OF DRAINAGE OF THE AXILLA AFTER LYMPH NODE DISSECTION
IN WOMEN WITH STAGE I OR II BREAST CANCER Protocol
PROTOCOL
IDS: RMNHS-1489, EU-20004
PROJECTED
ACCRUAL: A total of 200 patients will be accrued.
|
|
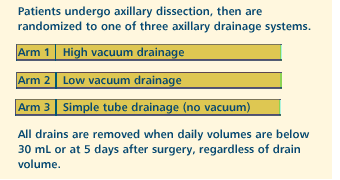
OBJECTIVE
- Compare high vacuum drainage vs low vacuum drainage vs
simple tube drainage in patients undergoing axillary surgery
for stage I or II breast cancer.
PARTICIPATION
CRITERIA
- Diagnosis
of resectable stage I or II breast cancer
- Planned
primary surgery of level II or III axillary dissection in
association with one of the following:
- Wide
local excision (may be done through separate incision)
- No
breast surgery
- No
immediate breast reconstruction using implants, latissimus
dorsi or rectus abdominus myocutaneous flaps at primary
operation
- No
prior axillary surgery
STUDY
CONTACTS
Gerald
Gui, Chair, Ph: +44-20-7808-2783
Royal Marsden NHS Trust
London, England, United Kingdom
Anthony
G. Nash, Ph: 0181-642-6011
Royal Marsden Hospital
Sutton, England, United Kingdom
N.P.M.
Sacks, Ph: 0207-808-2782
Royal Marsden NHS Trust
London, England, United Kingdom
|
| PHASE
II STUDY OF SENTINEL NODE BIOPSY TO ASSESS AXILLARY NODAL STATUS
IN PATIENTS WITH RESECTABLE STAGE I OR II BREAST CANCER Protocol
PROTOCOL
IDS: RMNHS-1631, EU-20006
PROJECTED
ACCRUAL: A total of 150 patients (75 per stratum) will
be accrued for the main study plus another 50 patients for
the pilot study.
|
|
OBJECTIVE
- Compare sentinel node biopsy vs axillary dissection in
determining axillary nodal status in patients with respectable
stage I or II breast cancer.
PARTICIPATION
CRITERIA
- Diagnosis
of stage I or II invasive breast cancer by triple assessment
(clinical, mammogram and/or ultrasound, FNA)
- Resectable
disease by either wide local excision or mastectomy with
axillary dissection
PROTOCOL
Patients
undergo lymphoscintigraphy, which consists of technetium Tc
99m human serum albumin colloid being injected near the tumor.
Dynamic imaging using a gamma camera is performed for 20 minutes
postinjection and static images are obtained for up to 3 hours
postinjection.
Surgery
is performed within 24 hours of lymphoscintigraphy. Patients
are injected with patent blue V dye near the tumor and a gamma
detection probe is used to measure radioactive counts in the
sentinel node. Surgery begins within 5 minutes of the patent
blue V dye injection.
All lymph
nodes that stain blue or have a high radioactive count are
removed. The primary breast lump is removed by either wide
local excision or mastectomy and the axilla are cleared by
standard axillary dissection. Some patients may only receive
patent blue V dye injected as a pilot study. Sentinel lymph
node biopsy and axillary dissection proceed as above.
STUDY
CONTACTS
Gerald
Gui, Chair, Ph: +44-20-7808-2783
Royal Marsden NHS Trust
London, England, United Kingdom
|
Page
2 of 2
Back | Select Publications
Top
Additional
Sections:
1 | 2
| 3 | 4
| 5 | 6
| 7 | 8
| 9 | 10
| 11 | 12
|
|
|

