
 |
||||||||

| Tracks 1-6 | ||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Would you discuss the new adjuvant trial being conducted
by the NSABP and CIRG evaluating trastuzumab with or without
bevacizumab?
DR LOVE: Would you discuss the new adjuvant trial being conducted
by the NSABP and CIRG evaluating trastuzumab with or without
bevacizumab?
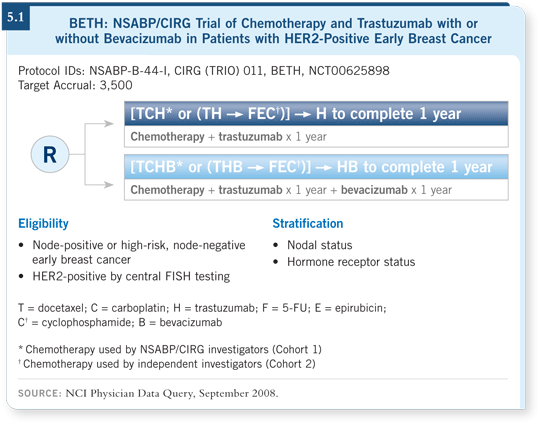
![]() DR WOLMARK: The BETH study opened recently (5.1). I believe we need
to know what the addition of bevacizumab to trastuzumab will yield in the
adjuvant setting, based on some interesting preclinical work and early clinical
findings (Pegram 2006).
DR WOLMARK: The BETH study opened recently (5.1). I believe we need
to know what the addition of bevacizumab to trastuzumab will yield in the
adjuvant setting, based on some interesting preclinical work and early clinical
findings (Pegram 2006).
![]() DR LOVE: What are the cardiac issues with this combination? Is the main
cardiovascular issue with bevacizumab hypertension?
DR LOVE: What are the cardiac issues with this combination? Is the main
cardiovascular issue with bevacizumab hypertension?
![]() DR WOLMARK: Both agents are concerns. The NSABP and the CIRG are
offering TCH as the template. We made the decision not to use an anthracycline
template to test the combination of trastuzumab and bevacizumab, with
one of the rationales being the potential toxicity of using both agents on an
anthracycline template. However, some participating physicians, particularly
those in Europe, will administer an anthracycline template along with bevacizumab and trastuzumab, so I believe we will receive an answer rapidly as to
whether that regimen is tolerable.
DR WOLMARK: Both agents are concerns. The NSABP and the CIRG are
offering TCH as the template. We made the decision not to use an anthracycline
template to test the combination of trastuzumab and bevacizumab, with
one of the rationales being the potential toxicity of using both agents on an
anthracycline template. However, some participating physicians, particularly
those in Europe, will administer an anthracycline template along with bevacizumab and trastuzumab, so I believe we will receive an answer rapidly as to
whether that regimen is tolerable.
Track 2
![]() DR LOVE: What’s your take on the controversy about the use of adjuvant
anthracyclines in HER2-negative disease?
DR LOVE: What’s your take on the controversy about the use of adjuvant
anthracyclines in HER2-negative disease?
![]() DR WOLMARK: I have some deep-seated thoughts on this issue. I believe that
the retreat from and abandonment of anthracyclines is proceeding with vigor
and with some degree of mysticism. However, we don’t have the definitive
data to abandon anthracyclines.
DR WOLMARK: I have some deep-seated thoughts on this issue. I believe that
the retreat from and abandonment of anthracyclines is proceeding with vigor
and with some degree of mysticism. However, we don’t have the definitive
data to abandon anthracyclines.
Track 3
![]() DR LOVE: Would you discuss the new collaboration between the NSABP
and US Oncology on the TC-TAC-TC/bevacizumab study?
DR LOVE: Would you discuss the new collaboration between the NSABP
and US Oncology on the TC-TAC-TC/bevacizumab study?
![]() DR WOLMARK: Sarah Cannon and US Oncology are evaluating six cycles of
TAC versus six cycles of TC, but will that be a definitive trial? The target
sample size is 2,000 patients, and the study has 80 percent power to detect a
3.4 percent absolute difference in disease-free survival in favor of the anthracycline.
What if the difference is only three percent and the p-value is 0.08?
What conclusions will we derive?
DR WOLMARK: Sarah Cannon and US Oncology are evaluating six cycles of
TAC versus six cycles of TC, but will that be a definitive trial? The target
sample size is 2,000 patients, and the study has 80 percent power to detect a
3.4 percent absolute difference in disease-free survival in favor of the anthracycline.
What if the difference is only three percent and the p-value is 0.08?
What conclusions will we derive?
So the NSABP, along with Steve Jones and US Oncology, would like to fold that trial into the “TIC-TAC-TOE” trial, or the 3T trial, in which we’re comparing TAC to TC to TC/bevacizumab in 3,900 patients (5.3).
We hope the last arm will determine whether bevacizumab on a nonanthracycline template can add benefit, and it will increase the sample size for the pairwise comparison of TAC to TC to approximately 3,600, which would provide more power to determine the value of an anthracycline or the lack thereof in a HER2-negative cohort.
Track 4
![]() DR LOVE: Can you discuss the letter to the editor in The New England
Journal of Medicine about the effects of adjuvant trastuzumab in “HER2-
low” tumors that Soon Paik and you published recently?
DR LOVE: Can you discuss the letter to the editor in The New England
Journal of Medicine about the effects of adjuvant trastuzumab in “HER2-
low” tumors that Soon Paik and you published recently?
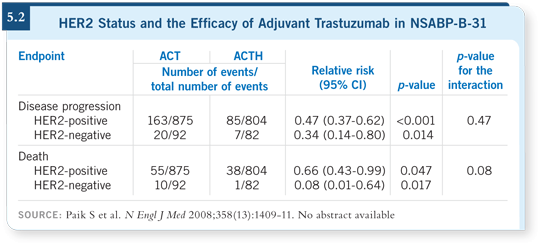
![]() DR WOLMARK: This work by Soon has far-reaching ramifications that I
believe challenge some of the concepts that many people thought were inviolate.
In evaluating the 500 or so patients in NSABP-B-31, who on review
were not IHC3+ and were not FISH-positive using the standard criteria (5.2),
the forest plots indicate little difference in benefit of trastuzumab between
those who were HER2-low and those who were HER2-positive.
DR WOLMARK: This work by Soon has far-reaching ramifications that I
believe challenge some of the concepts that many people thought were inviolate.
In evaluating the 500 or so patients in NSABP-B-31, who on review
were not IHC3+ and were not FISH-positive using the standard criteria (5.2),
the forest plots indicate little difference in benefit of trastuzumab between
those who were HER2-low and those who were HER2-positive.
Dr Paik exhaustively analyzed this using a number of methodologies — with expression and with mRNA-based assays. Consistently, those individuals with HER2-low disease on IHC or FISH had HER2-low disease in terms of expression also. He went so far as to evaluate genes that were adjacent to HER2, and if the HER2 level was low, the levels of adjacent genes were also low. We’re confident that this is not a misinterpretation of morphology, IHC or FISH analysis — this is real (5.4).
We submitted to CTEP a concept, which has been accepted pending requirements, for addressing the trastuzumab question in this HER2-low subset — IHC1+, IHC2+ and FISH-negative — which accounts for 40 percent of patients and is not a trivial number.
We submitted the concept of a trial looking at TC (docetaxel/cyclophosphamide) versus TC/trastuzumab for patients with HER2-low, high-risk, node-negative and node-positive breast cancer.
CTEP first required a blinded round-robin review of the slides, IHC and FISH, by three objective pathologists. If their findings are in concordance with the NSABP pathology findings, then this trial will move forward.

Track 6
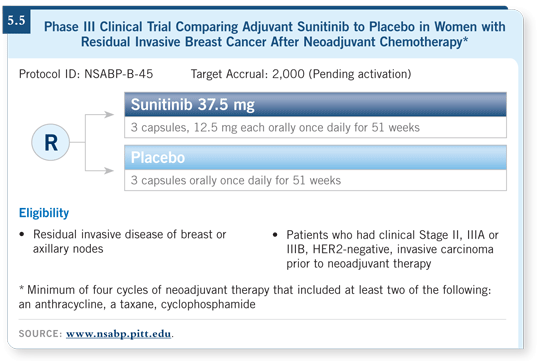
![]() DR LOVE: Another study I want to ask you about is the new NSABP-B-
45 study, evaluating patients with residual tumor after neoadjuvant anthracycline/taxane therapy.
DR LOVE: Another study I want to ask you about is the new NSABP-B-
45 study, evaluating patients with residual tumor after neoadjuvant anthracycline/taxane therapy.
![]() DR WOLMARK: This trial will evaluate patients considered to be at high risk
based on the observation that they did not achieve a pathologic complete response,
either in the primary breast or in the axillary nodes, after preoperative therapy.
DR WOLMARK: This trial will evaluate patients considered to be at high risk
based on the observation that they did not achieve a pathologic complete response,
either in the primary breast or in the axillary nodes, after preoperative therapy.
Patients will be randomly assigned to one year of sunitinib or to placebo (5.5). This is an exciting setting in which to determine the value of a biologic agent for this patient population. Currently we do not have an algorithm to predict patient benefit in this particular subset, so robust tissue collection will be a prerequisite as we evaluate possible predictive markers for likelihood of patient benefit from sunitinib therapy.
EDITOR
Neil Love, MD
INTERVIEWS
Nancy E Davidson, MD
- Select publications
Professor John Crown, MD
- Select publications
Kathy D Miller, MD
- Select publications
Peter M Ravdin, MD, PhD
- Select publications
THREE PERSPECTIVES ON US COOPERATIVE GROUP RESEARCH
Norman Wolmark, MD
- Select publications
Joyce O’Shaughnessy, MD
- Select publications
Eric P Winer, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity