
 |
||||||||

| Tracks 1-11 | ||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: In your ECOG-E5103 trial, what were the safety issues relative
to evaluating bevacizumab in the adjuvant setting?
DR LOVE: In your ECOG-E5103 trial, what were the safety issues relative
to evaluating bevacizumab in the adjuvant setting?
![]() DR MILLER: When designing trials in the adjuvant setting, we had to consider
whether unique safety concerns existed for bevacizumab. Even in the adjuvant
trials for patients at the highest risk, more than half of the patients in the
control groups fare well, and many do so with no systemic therapy. So toxicities
that might be rare and of no concern in the metastatic setting are a bigger
concern in the adjuvant setting.
DR MILLER: When designing trials in the adjuvant setting, we had to consider
whether unique safety concerns existed for bevacizumab. Even in the adjuvant
trials for patients at the highest risk, more than half of the patients in the
control groups fare well, and many do so with no systemic therapy. So toxicities
that might be rare and of no concern in the metastatic setting are a bigger
concern in the adjuvant setting.
We believed that most of the bevacizumab toxicities were not likely to be major issues. Arterial thrombotic events are rare in the metastatic population: We expect them to be even more rare in the healthier, younger adjuvant population. The venous thromboembolic events are also likely to be uncommon and certainly not prohibitive. Proteinuria is a fairly rare toxicity to be of any clinical importance, and it improves with time off therapy.
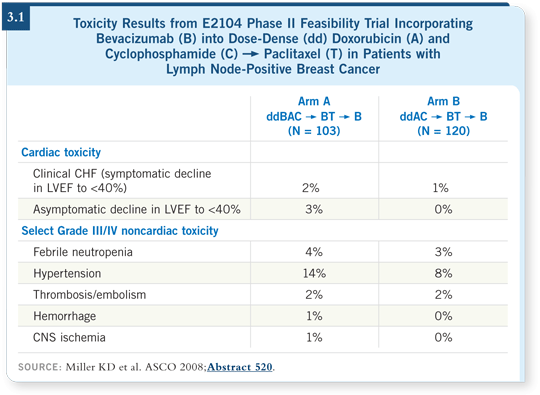
With bevacizumab in the adjuvant setting, I am most concerned about hypertension, but that is a long-term concern that may not become apparent for 10, 15 or 30 years. We were concerned about cardiac toxicity. At the time we first started considering adjuvant therapy, reports existed of patients treated collectively in three separate trials in different settings with different anthracycline regimens, but they all raised the question of either clinical congestive heart failure or asymptomatic decreases in ejection fraction to levels that are of concern (lower than 40 percent).
With such small numbers, the confidence intervals were wide, and clinical event reports of congestive heart failure ranged from zero to 27 percent of patients (Swain 2003). That’s a big difference. If the incidence were zero, you would move forward with an adjuvant trial with little monitoring. If it were 27 percent, you would not move forward.
We designed a pilot trial (3.1) to make sure that the rates of clinically apparent congestive heart failure were not prohibitive. We agreed in advance that a clinical rate of 10 percent or more would be prohibitive. For the average patient, it would be unlikely that the benefits of therapy, if they existed, would outweigh that potential risk, and so we should examine other strategies.
Track 2
![]() DR LOVE: What is the design of the ECOG-E5103 trial?
DR LOVE: What is the design of the ECOG-E5103 trial?
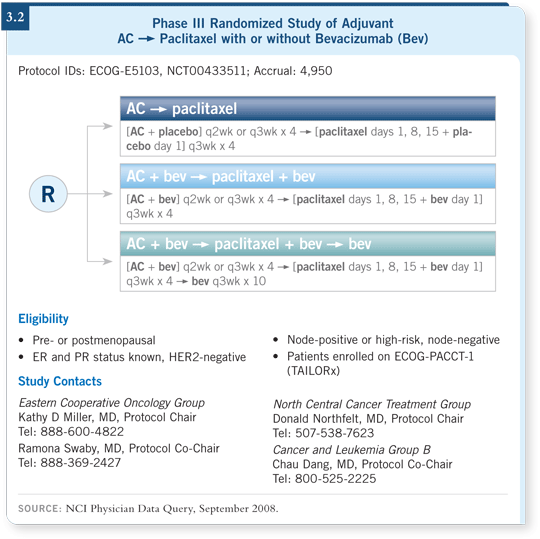
![]() DR MILLER: ECOG-E5103 is a large adjuvant study that encompasses several
features (3.2). It has a practical element in that we allow patients and their physicians to select administration of AC every two weeks or every three
weeks. We’ll stratify for that choice, so it won’t affect our results. This design
builds on the improvements we’ve made in adjuvant therapy. The backbone of
the chemotherapy is four cycles of AC followed by weekly paclitaxel. That’s
building on ECOG-E1199 (Sparano 2005), the adjuvant trastuzumab studies
and the E2100 trial in the metastatic setting (Miller 2007).
DR MILLER: ECOG-E5103 is a large adjuvant study that encompasses several
features (3.2). It has a practical element in that we allow patients and their physicians to select administration of AC every two weeks or every three
weeks. We’ll stratify for that choice, so it won’t affect our results. This design
builds on the improvements we’ve made in adjuvant therapy. The backbone of
the chemotherapy is four cycles of AC followed by weekly paclitaxel. That’s
building on ECOG-E1199 (Sparano 2005), the adjuvant trastuzumab studies
and the E2100 trial in the metastatic setting (Miller 2007).
It also incorporates preclinical data on the potential synergy between lower-dose but more continuous taxane exposure and bevacizumab. In laboratory studies, at doses much lower than the doses that are required to have any direct cytotoxic effect on the tumor cells, the taxanes have a separate effect on endothelial cells (Ng 2004). To obtain that effect, however, you need prolonged exposure. With weekly schedules, we have a lower dose but more continuous exposure to the drug.
The entry criteria are different from what has been seen: We allow patients with node-negative disease but who are at high risk. We consider anyone with ER-negative disease and a tumor larger than one centimeter to be at increased risk. Patients with ER-positive, node-negative disease are considered at high risk only if the tumors are larger than five centimeters or if they are between one and five centimeters and the Oncotype DX® Recurrence Score® is not low. We chose the Oncotype DX cutoff as 11 to match TAILORx. If a patient on TAILORx has a high- or intermediate-risk score and is assigned to chemotherapy, she’s welcome to participate in E5103 as a way to receive chemotherapy.
Track 3
![]() DR LOVE: Based on your perspective on the mechanism of action of
bevacizumab, are you expecting it to be active in the adjuvant setting?
DR LOVE: Based on your perspective on the mechanism of action of
bevacizumab, are you expecting it to be active in the adjuvant setting?
![]() DR MILLER: I believe that bevacizumab will be effective, but hypotheses with
evidence exist on both sides of the question. Angiogenesis may be regarded as
one of the earliest events that a tumor cell must accomplish. We see evidence
of angiogenesis even in DCIS, in which the tumors are not yet invasive. It is
an early phenomenon, which suggests that adjuvant therapy might be effective.
Studies examining the expression of pro-angiogenic factors in atypical (but not
yet malignant) lesions, DCIS and invasive disease show that more angiogenic
factors are expressed as the tumors become older. These observations suggest
that agents like bevacizumab might be more effective earlier in the course of
the disease, which would bring you into the adjuvant setting.
DR MILLER: I believe that bevacizumab will be effective, but hypotheses with
evidence exist on both sides of the question. Angiogenesis may be regarded as
one of the earliest events that a tumor cell must accomplish. We see evidence
of angiogenesis even in DCIS, in which the tumors are not yet invasive. It is
an early phenomenon, which suggests that adjuvant therapy might be effective.
Studies examining the expression of pro-angiogenic factors in atypical (but not
yet malignant) lesions, DCIS and invasive disease show that more angiogenic
factors are expressed as the tumors become older. These observations suggest
that agents like bevacizumab might be more effective earlier in the course of
the disease, which would bring you into the adjuvant setting.
I also have questions about the duration of therapy. We administer bevacizumab for two durations in E5103: approximately six months and approximately one year. Perhaps that’s not long enough. Perhaps you need chronic therapy, not to eliminate microscopic disease but rather to keep it from growing. If we remove that foot from the brake, we may prolong time to progression but perhaps not prevent recurrence or change overall survival.
Track 5
![]() DR LOVE: Would you describe what was found in the AVADO trial?
DR LOVE: Would you describe what was found in the AVADO trial?
![]() DR MILLER: AVADO was the European equivalent of my E2100 trial with an
important addition. AVADO had three arms: docetaxel alone at the European-favored
100-mg/m2 dose with placebo, docetaxel and bevacizumab at 7.5 milligrams
per kilogram every three weeks (half the dose we typically use in breast
cancer studies) or docetaxel and bevacizumab at 15 milligrams per kilogram.
DR MILLER: AVADO was the European equivalent of my E2100 trial with an
important addition. AVADO had three arms: docetaxel alone at the European-favored
100-mg/m2 dose with placebo, docetaxel and bevacizumab at 7.5 milligrams
per kilogram every three weeks (half the dose we typically use in breast
cancer studies) or docetaxel and bevacizumab at 15 milligrams per kilogram.
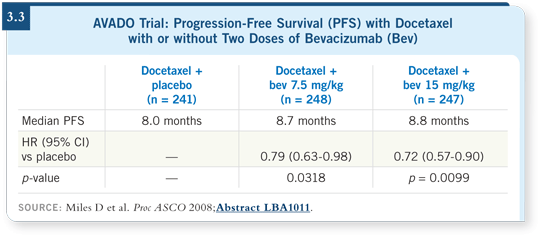
It wasn’t designed to compare the two bevacizumab arms but to effect two pairwise comparisons: low-dose bevacizumab versus placebo and high-dose bevacizumab versus placebo. We saw statistically significant improvements in response rates and progression-free survival with bevacizumab, but in absolute terms it was disappointing (Miles 2008). In the control group, progression-free survival was eight months. It increased to 8.7 months for patients in the low-dose bevacizumab group and to 8.8 months in the high-dose group (Miles 2008; [3.3]).
It’s clear that the curves separate early and remain separate throughout most of the follow-up period. This is a real difference and a bigger difference than the roughly one-month medians might suggest. But 8.8 months is still not 11.8 months. With the high dose and the intermittent schedule, we wouldn’t predict that the docetaxel regimen would take advantage of the potential anti-angiogenic activity of the taxanes.
I’m even more interested in the future results of the RIBBON 1 trial, which questions the assumption that you can add bevacizumab to any chemotherapy and obtain the same results. Particular drugs and schedules may be much more synergistic and offer a greater benefit for the combination than what you would obtain with others.
EDITOR
Neil Love, MD
INTERVIEWS
Nancy E Davidson, MD
- Select publications
Professor John Crown, MD
- Select publications
Kathy D Miller, MD
- Select publications
Peter M Ravdin, MD, PhD
- Select publications
THREE PERSPECTIVES ON US COOPERATIVE GROUP RESEARCH
Norman Wolmark, MD
- Select publications
Joyce O’Shaughnessy, MD
- Select publications
Eric P Winer, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity