
 |
||||||||

| Tracks 1-4 | ||||||||||
|
Select Excerpts from the Interview
Tracks 1-2
![]() DR LOVE: What’s your take on tissue biomarkers and the design of new
clinical trials?
DR LOVE: What’s your take on tissue biomarkers and the design of new
clinical trials?
![]() DR WINER: HER2-positive disease is clearly separate from everything else
at this point, and I believe that’s truer in 2008 than it was in 2004 or 2002.
With the recognition from the large randomized trials that trastuzumab works
in the adjuvant setting (Joensuu 2006; Romond 2005; Slamon 2006; Smith
2007) and with the development of post-trastuzumab therapies like lapatinib
(Di Leo 2007; Geyer 2006; O’Shaughnessy 2008), I believe that in both
the adjuvant setting and the metastatic setting we will have HER2-positive
trials and HER2-negative trials. With the exception of some Phase I trials or
perhaps some limited other examples, we will not see a great deal of mixing.
DR WINER: HER2-positive disease is clearly separate from everything else
at this point, and I believe that’s truer in 2008 than it was in 2004 or 2002.
With the recognition from the large randomized trials that trastuzumab works
in the adjuvant setting (Joensuu 2006; Romond 2005; Slamon 2006; Smith
2007) and with the development of post-trastuzumab therapies like lapatinib
(Di Leo 2007; Geyer 2006; O’Shaughnessy 2008), I believe that in both
the adjuvant setting and the metastatic setting we will have HER2-positive
trials and HER2-negative trials. With the exception of some Phase I trials or
perhaps some limited other examples, we will not see a great deal of mixing.
I believe that classifying breast cancer into clinically relevant subtypes will allow us, and has already allowed us, to design trials that will lead to far more tangible benefits than in the past. We’ve recently begun to see the results of that with trials of trastuzumab in the adjuvant setting. Now it must be taken several steps further.
We need more work conducted for patients with triple-negative disease. At the moment we have chemotherapy and the added benefits of bevacizumab in the metastatic setting but not much else. I believe that for patients with ER-positive, HER2-negative disease, the key is identifying who will benefit from chemotherapy and who will not. We also need to figure out how to better utilize hormonal therapy.
We have good clues, and they relate largely to the duration of therapy and potentially selecting the right drug for the right patient rather than believing the same approach will work for everyone.
![]() DR LOVE: Do you think that type of research strategy will result in a significant
reduction in breast cancer mortality in the next 10 or 15 years?
DR LOVE: Do you think that type of research strategy will result in a significant
reduction in breast cancer mortality in the next 10 or 15 years?
![]() DR WINER: I believe adjuvant HER2-directed therapy will ultimately result
in close to a 50 percent reduction in the deaths from HER2-positive breast
cancer among women who present with early-stage breast cancer.
DR WINER: I believe adjuvant HER2-directed therapy will ultimately result
in close to a 50 percent reduction in the deaths from HER2-positive breast
cancer among women who present with early-stage breast cancer.
At the moment, the treatment for those patients is trastuzumab, but we may evolve beyond that. I’m phrasing it that way deliberately because a fair number of women with HER2-positive breast cancer, unfortunately, still present with advanced disease.
In another seven or eight years, I believe we will have almost all, if not all, the tools we need to eliminate death from HER2-positive breast cancer.
Tracks 3-4
![]() DR LOVE: Do you have any predictions about how the “HER2-low”
issue will play out in terms of benefit from adjuvant trastuzumab?
DR LOVE: Do you have any predictions about how the “HER2-low”
issue will play out in terms of benefit from adjuvant trastuzumab?
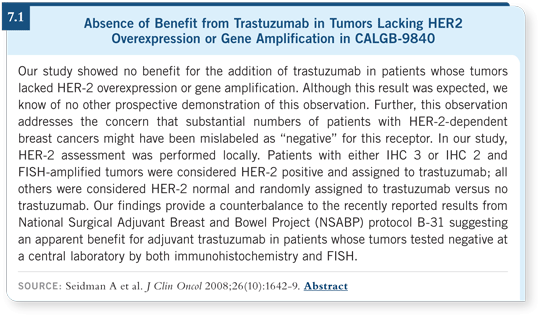
![]() DR WINER: What troubles all of us about Soon Paik’s data (Paik 2007, 2008;
[5.2]) is that everything we know in the metastatic setting indicates that
patients with disease not classified as HER2-positive by our current standards
— FISH-positive or IHC 3+ — do not benefit from trastuzumab (7.1).
DR WINER: What troubles all of us about Soon Paik’s data (Paik 2007, 2008;
[5.2]) is that everything we know in the metastatic setting indicates that
patients with disease not classified as HER2-positive by our current standards
— FISH-positive or IHC 3+ — do not benefit from trastuzumab (7.1).
Maybe trastuzumab is working in a different way in the adjuvant setting, but that seems to be a stretch. I’m open to the possibilities, but I need a lot more data before concluding that trastuzumab or other HER2-directed therapy has a role for patients whose tumors we believe are HER2-negative.
I believe that the next step should be a study of patients whose tumors are considered HER2-intermediate — defined as 1.8 to 2.2 by FISH. I’d also consider IHC 2+ without anything else, for that matter. No one knows what to do with those patients. We should enroll them in a study and answer the question.
![]() DR LOVE: What about IHC 1+?
DR LOVE: What about IHC 1+?
![]() DR WINER: Patients in the NSABP-B-31 trial all had HER2 that was defined
as positive somewhere. It would seem easier to justify the low risk and the
added burden associated with trastuzumab for those patients with IHC 2+
readings. Considering the benefits that have been reported with trastuzumab,
including the benefits in this small subset, it wouldn’t require a huge trial to
find out.
DR WINER: Patients in the NSABP-B-31 trial all had HER2 that was defined
as positive somewhere. It would seem easier to justify the low risk and the
added burden associated with trastuzumab for those patients with IHC 2+
readings. Considering the benefits that have been reported with trastuzumab,
including the benefits in this small subset, it wouldn’t require a huge trial to
find out.
EDITOR
Neil Love, MD
INTERVIEWS
Nancy E Davidson, MD
- Select publications
Professor John Crown, MD
- Select publications
Kathy D Miller, MD
- Select publications
Peter M Ravdin, MD, PhD
- Select publications
THREE PERSPECTIVES ON US COOPERATIVE GROUP RESEARCH
Norman Wolmark, MD
- Select publications
Joyce O’Shaughnessy, MD
- Select publications
Eric P Winer, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity