
 |
||||||||

| Tracks 1-11 | ||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 2-3, 5
![]() DR LOVE: Would you discuss the similarities and differences between the
Oncotype DX and MammaPrint assays?
DR LOVE: Would you discuss the similarities and differences between the
Oncotype DX and MammaPrint assays?
![]() DR RAVDIN: Each of these two tests provides a molecular profile based on
RNA. In theory, they’re similar. In practice, however, they’re quite different.
The Oncotype DX assay, also called the 21-gene assay, analyzes fragments of
mRNA in archived tissue. The assay is performed on paraffin-embedded,
fixed tumor tissue. This attribute opens up rapid development of the assay
because most of the large cooperative groups have been collecting block
materials for more than a decade, back into the 1980s. So this test has an
enormous advantage in terms of development.
DR RAVDIN: Each of these two tests provides a molecular profile based on
RNA. In theory, they’re similar. In practice, however, they’re quite different.
The Oncotype DX assay, also called the 21-gene assay, analyzes fragments of
mRNA in archived tissue. The assay is performed on paraffin-embedded,
fixed tumor tissue. This attribute opens up rapid development of the assay
because most of the large cooperative groups have been collecting block
materials for more than a decade, back into the 1980s. So this test has an
enormous advantage in terms of development.
We also have the 70-gene test, or MammaPrint assay, which is dependent on intact mRNA. Because the large clinical trials haven’t historically banked intact tissue, MammaPrint requires samples to be frozen or specially preserved in alcohol. This assay is dependent on institutional series, in which the therapy has not been standardized as it has been in cooperative group trials.
![]() DR LOVE: Do data exist with MammaPrint predicting benefit from chemotherapy
as with Oncotype DX?
DR LOVE: Do data exist with MammaPrint predicting benefit from chemotherapy
as with Oncotype DX?
![]() DR RAVDIN: No. The problem is the lack of a good comparison group. The
MammaPrint assay requires fresh tissue, and all of the data are focused on
prognosis.
DR RAVDIN: No. The problem is the lack of a good comparison group. The
MammaPrint assay requires fresh tissue, and all of the data are focused on
prognosis.
That’s the genesis of the prospective MINDACT trial, in which patients are being randomly assigned to receive chemotherapy or not (Cardoso 2008). Those patients are undergoing MammaPrint profiles, and the study results should tell us what we already know for the Oncotype DX test.
Two studies have already reported results on Oncotype DX. NSABP-B-20 randomly assigned patients with node-negative disease to tamoxifen or tamoxifen with CMF — also, some patients received MF in that trial (Paik 2006).
Late last year, Oncotype results were reported for SWOG-8814, which randomly assigned postmenopausal patients with ER-positive, node-positive disease to tamoxifen alone or tamoxifen with CAF (Albain 2007; [4.1]).
![]() DR LOVE: In Albain’s study with Oncotype DX, the baseline risk of recurrence
for patients with node-positive disease — even those in the low Recurrence
Score group — was substantial. However, patients with a low Recurrence
Score did not appear to benefit from chemotherapy (Albain 2007; [4.1]).
DR LOVE: In Albain’s study with Oncotype DX, the baseline risk of recurrence
for patients with node-positive disease — even those in the low Recurrence
Score group — was substantial. However, patients with a low Recurrence
Score did not appear to benefit from chemotherapy (Albain 2007; [4.1]).
![]() DR RAVDIN: With classic pathology we have not been able to predict benefit
from chemotherapy, whereas the genetic profiles across studies consistently
show that the patients with low-risk genetic profiles do not benefit from
chemotherapy (Paik 2006; Albain 2007). In trials that have reported clear
benefit from chemotherapy in one or more arms, the benefit has been for
patients with high Oncotype DX Recurrence Scores. I believe the jury is still out regarding the patients with intermediate Oncotype DX Recurrence Scores.
DR RAVDIN: With classic pathology we have not been able to predict benefit
from chemotherapy, whereas the genetic profiles across studies consistently
show that the patients with low-risk genetic profiles do not benefit from
chemotherapy (Paik 2006; Albain 2007). In trials that have reported clear
benefit from chemotherapy in one or more arms, the benefit has been for
patients with high Oncotype DX Recurrence Scores. I believe the jury is still out regarding the patients with intermediate Oncotype DX Recurrence Scores.
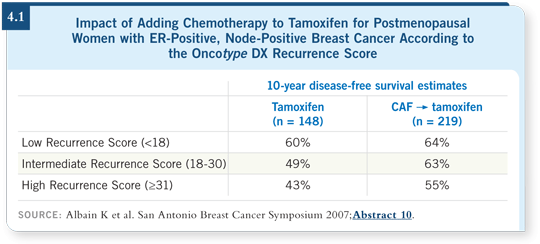
As a clinician, if you’re evaluating a patient for whom you’re undecided about treating on the basis of prognosis and you note that she has a low Oncotype DX Recurrence Score, then an additional piece of information that may strongly sway you is the fact that substantial clinical evidence from these two studies, performed in somewhat different populations with different chemotherapeutic regimens, indicates that those patients don’t benefit from either CMF or CAF (Paik 2006; Albain 2007). This doesn’t cover the entire spectrum of questions that might be asked, but it’s a consistent story that those patients don’t benefit from chemotherapy.
Track 8
![]() DR LOVE: Where do you think we are headed in terms of the measurement
of ER and HER2? Is RT-PCR the future?
DR LOVE: Where do you think we are headed in terms of the measurement
of ER and HER2? Is RT-PCR the future?
![]() DR RAVDIN: I believe so. It’s useful because we’ve had numerous indications
that ER level does help predict response to tamoxifen, and I’ve seen suggestions
that it may eventually refine prediction of responsiveness to chemotherapy also.
DR RAVDIN: I believe so. It’s useful because we’ve had numerous indications
that ER level does help predict response to tamoxifen, and I’ve seen suggestions
that it may eventually refine prediction of responsiveness to chemotherapy also.
![]() DR LOVE: Can you envision a situation in which the quantitative ER assessment
might drive decisions in metastatic disease?
DR LOVE: Can you envision a situation in which the quantitative ER assessment
might drive decisions in metastatic disease?
![]() DR RAVDIN: Yes, I believe that it would be useful in the treatment of
metastatic disease. So often in a clinical situation, you don’t want to waste
weeks waiting for hormonal therapy to work unless you are confident that the
patient will respond.
DR RAVDIN: Yes, I believe that it would be useful in the treatment of
metastatic disease. So often in a clinical situation, you don’t want to waste
weeks waiting for hormonal therapy to work unless you are confident that the
patient will respond.
Track 9
![]() DR LOVE: What is your take on the plenary presentation of ABCSG-12 at
ASCO 2008 by Mike Gnant (Gnant 2008) reporting a 35 percent reduction
in relapse rate in women who received adjuvant zoledronic acid?
DR LOVE: What is your take on the plenary presentation of ABCSG-12 at
ASCO 2008 by Mike Gnant (Gnant 2008) reporting a 35 percent reduction
in relapse rate in women who received adjuvant zoledronic acid?
![]() DR RAVDIN: It was a spectacular effect. In addition, we’ve seen hints of it in
other trials. In this case, however, they administered a strong IV bisphosphonate
every six months. This is another trastuzumab in that it is similar to the
magnitude of benefit seen with adjuvant trastuzumab.
DR RAVDIN: It was a spectacular effect. In addition, we’ve seen hints of it in
other trials. In this case, however, they administered a strong IV bisphosphonate
every six months. This is another trastuzumab in that it is similar to the
magnitude of benefit seen with adjuvant trastuzumab.
However, it’s different from the trastuzumab story in that we don’t have three trials reported at the same meeting. I hope these data are corroborated because a 35 percent reduction in relapse rate from a drug whose major side effect is that you don’t become osteopenic would be wonderful.
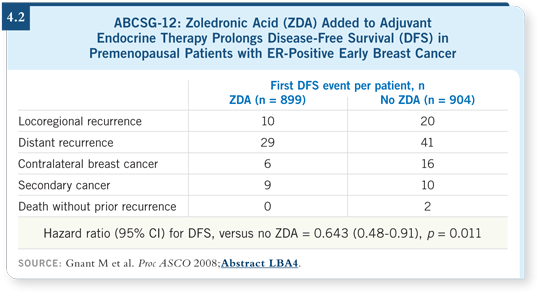
![]() DR LOVE: It was interesting that the rates of contralateral disease, local recurrence
and distant metastasis — even nonbone metastasis — were lower among
patients receiving zoledronic acid (4.2).
DR LOVE: It was interesting that the rates of contralateral disease, local recurrence
and distant metastasis — even nonbone metastasis — were lower among
patients receiving zoledronic acid (4.2).
![]() DR RAVDIN: That is enormously important, suggesting an effect on visceral disease also, which was surprising. It may be broader than simply a local bone
effect. We will have more information about this soon. The NSABP-B-34
study evaluating adjuvant clodronate with or without chemotherapy and/or
hormonal therapy is closed and will probably report soon.
DR RAVDIN: That is enormously important, suggesting an effect on visceral disease also, which was surprising. It may be broader than simply a local bone
effect. We will have more information about this soon. The NSABP-B-34
study evaluating adjuvant clodronate with or without chemotherapy and/or
hormonal therapy is closed and will probably report soon.
![]() DR LOVE: In terms of clinical decision-making today, assuming reimbursement
is not an issue, is this reasonable to recommend to patients as an option,
or should we wait?
DR LOVE: In terms of clinical decision-making today, assuming reimbursement
is not an issue, is this reasonable to recommend to patients as an option,
or should we wait?
![]() DR RAVDIN: If a patient is receiving an aromatase inhibitor and you’re already
unsure whether or not you should treat, I believe that this story becomes
compelling and that those patients should be treated with a bisphosphonate.
Perhaps before, if a patient had mild osteopenia, we would simply observe, and
if it worsened, we would begin bisphosphonate treatment. These results argue
that you should probably start treating those patients earlier. The study was
performed with premenopausal patients, but I believe that the argument that
they’re essentially postmenopausal after the ovarian suppression is convincing.
DR RAVDIN: If a patient is receiving an aromatase inhibitor and you’re already
unsure whether or not you should treat, I believe that this story becomes
compelling and that those patients should be treated with a bisphosphonate.
Perhaps before, if a patient had mild osteopenia, we would simply observe, and
if it worsened, we would begin bisphosphonate treatment. These results argue
that you should probably start treating those patients earlier. The study was
performed with premenopausal patients, but I believe that the argument that
they’re essentially postmenopausal after the ovarian suppression is convincing.
EDITOR
Neil Love, MD
INTERVIEWS
Nancy E Davidson, MD
- Select publications
Professor John Crown, MD
- Select publications
Kathy D Miller, MD
- Select publications
Peter M Ravdin, MD, PhD
- Select publications
THREE PERSPECTIVES ON US COOPERATIVE GROUP RESEARCH
Norman Wolmark, MD
- Select publications
Joyce O’Shaughnessy, MD
- Select publications
Eric P Winer, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity