
 |
||||||||

| Tracks 1-14 | ||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 5
![]() DR LOVE: Would you discuss the trial you presented at ASCO, combining
trastuzumab and lapatinib in patients with HER2-positive metastatic disease?
DR LOVE: Would you discuss the trial you presented at ASCO, combining
trastuzumab and lapatinib in patients with HER2-positive metastatic disease?
![]() DR O’SHAUGHNESSY: This study consisted of patients who were heavily
pretreated for metastatic disease. Prior treatments included an average of
three trastuzumab-based regimens and a median of four to five chemotherapy
regimens. Twenty-five percent of the patients had received 10 or more treatments.
In addition, patients were required to have already experienced disease
progression through an anthracycline and a taxane and at least one trastuzumab-based regimen, and they must have been experiencing progression on
trastuzumab at study entry.
DR O’SHAUGHNESSY: This study consisted of patients who were heavily
pretreated for metastatic disease. Prior treatments included an average of
three trastuzumab-based regimens and a median of four to five chemotherapy
regimens. Twenty-five percent of the patients had received 10 or more treatments.
In addition, patients were required to have already experienced disease
progression through an anthracycline and a taxane and at least one trastuzumab-based regimen, and they must have been experiencing progression on
trastuzumab at study entry.
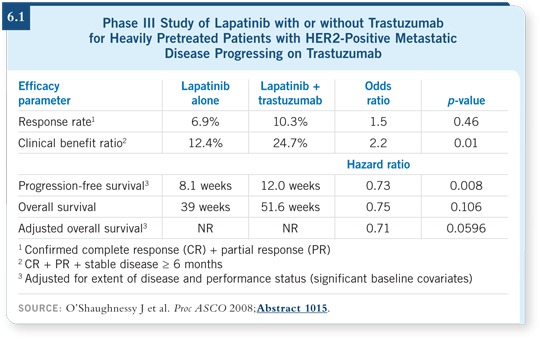
Approximately 300 patients were randomly assigned to lapatinib alone at 1,500 milligrams daily, or a lower dose at 1,000 milligrams daily, with weekly trastuzumab. The primary endpoint was progression-free survival, and the median increased from eight weeks with monotherapy to 12 weeks with the combination (O’Shaughnessy 2008; [6.1]). That was statistically significant, with a hazard ratio of 0.75.
An increase of four weeks may not seem that impressive, but the proportion of patients who were progression free at six months — which I believe is more important clinically — doubled, from 13 percent with lapatinib to 28 percent with lapatinib/trastuzumab. In addition, the survival data were almost significant once adjusted for performance status and extent of disease.
Continuing the trastuzumab and adding lapatinib was better for patients, and it was well tolerated. One implication is that lapatinib and trastuzumab appears to be a reasonable option for patients with metastatic disease indolent enough to take a chemotherapy holiday.
The other implication is that this double blockade of the HER2 pathway — blocking from the outside with trastuzumab and the inside with lapatinib — seems worthy of pursuit in additional clinical trials, such as the ALTTO adjuvant trial and other front-line and preoperative trials that are underway.
Track 6
![]() DR LOVE: What do you think of the combination of lapatinib and
capecitabine?
DR LOVE: What do you think of the combination of lapatinib and
capecitabine?
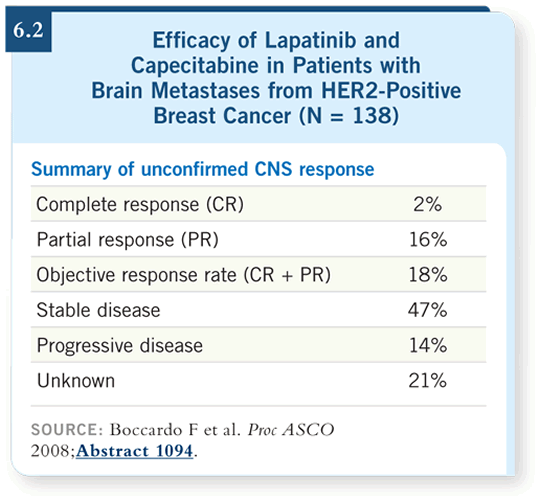
![]() DR O’SHAUGHNESSY: This is an important combination, particularly for
patients who have or are at high risk for developing brain metastases. At
ASCO 2008, Boccardo reported an 18 percent objective response rate among
patients who had definitive progressing brain metastases at the time of study
entry (Boccardo 2008; [6.2]). These data corroborated Lin and Winer’s
experience presented at the San Antonio Breast Cancer Symposium in 2007
(Lin 2007). The responses are impressive. Brain metastases are a scourge, and
we have so little to offer these patients other than radiation therapy. Thus I
am “bullish” on the capecitabine/lapatinib regimen as our most promising
strategy to help these patients, and I like to use it earlier in the metastatic
setting.
DR O’SHAUGHNESSY: This is an important combination, particularly for
patients who have or are at high risk for developing brain metastases. At
ASCO 2008, Boccardo reported an 18 percent objective response rate among
patients who had definitive progressing brain metastases at the time of study
entry (Boccardo 2008; [6.2]). These data corroborated Lin and Winer’s
experience presented at the San Antonio Breast Cancer Symposium in 2007
(Lin 2007). The responses are impressive. Brain metastases are a scourge, and
we have so little to offer these patients other than radiation therapy. Thus I
am “bullish” on the capecitabine/lapatinib regimen as our most promising
strategy to help these patients, and I like to use it earlier in the metastatic
setting.
![]() DR LOVE: What is your first-line regimen for patients with HER2-positive
metastatic disease?
DR LOVE: What is your first-line regimen for patients with HER2-positive
metastatic disease?
![]() DR O’SHAUGHNESSY: For patients who received adjuvant trastuzumab and
experienced at least a one-year disease-free interval since stopping the therapy,
I start with vinorelbine/trastuzumab. I like this combination for its efficacy
and quality of life. I then use capecitabine/lapatinib as my next line of therapy.
If a patient experiences toxicity with the lapatinib/capecitabine regimen, I can
easily imagine using lapatinib/trastuzumab based on the data I presented at
ASCO with this combination in heavily pretreated patients (O’Shaughnessy
2008).
DR O’SHAUGHNESSY: For patients who received adjuvant trastuzumab and
experienced at least a one-year disease-free interval since stopping the therapy,
I start with vinorelbine/trastuzumab. I like this combination for its efficacy
and quality of life. I then use capecitabine/lapatinib as my next line of therapy.
If a patient experiences toxicity with the lapatinib/capecitabine regimen, I can
easily imagine using lapatinib/trastuzumab based on the data I presented at
ASCO with this combination in heavily pretreated patients (O’Shaughnessy
2008).
![]() DR LOVE: Have you seen responses to the lapatinib/capecitabine regimen in
patients with brain metastases?
DR LOVE: Have you seen responses to the lapatinib/capecitabine regimen in
patients with brain metastases?
![]() DR O’SHAUGHNESSY:
I have seen minor responses,
but even more impressive,
I’ve seen prolonged stable
disease. For example, I have
patients who have undergone
whole-brain radiation
therapy and resection and
then received this combination
when they returned
with progressive disease. In
these patients, I have seen
prolonged disease control —
for more than a year and for
some patients even pushing
two years.
DR O’SHAUGHNESSY:
I have seen minor responses,
but even more impressive,
I’ve seen prolonged stable
disease. For example, I have
patients who have undergone
whole-brain radiation
therapy and resection and
then received this combination
when they returned
with progressive disease. In
these patients, I have seen
prolonged disease control —
for more than a year and for
some patients even pushing
two years.
Tracks 9-11
![]() DR LOVE: What was the rationale for combining bevacizumab with
the nonanthracycline regimen TC in the adjuvant setting on the US
Oncology/NSABP “TIC-TAC-TOE” trial (5.3)?
DR LOVE: What was the rationale for combining bevacizumab with
the nonanthracycline regimen TC in the adjuvant setting on the US
Oncology/NSABP “TIC-TAC-TOE” trial (5.3)?
![]() DR O’SHAUGHNESSY: The hypothesis is that a HER2-negative population
exists that does not need anthracyclines. Many groups are interested in that
hypothesis, including US Oncology, Sarah Cannon, TORI and the NSABP.
If indeed that is the case, then we want to see what bevacizumab contributes
to a nonanthracycline regimen. This is similar to the BETH trial approach
examining TCH and bevacizumab.
DR O’SHAUGHNESSY: The hypothesis is that a HER2-negative population
exists that does not need anthracyclines. Many groups are interested in that
hypothesis, including US Oncology, Sarah Cannon, TORI and the NSABP.
If indeed that is the case, then we want to see what bevacizumab contributes
to a nonanthracycline regimen. This is similar to the BETH trial approach
examining TCH and bevacizumab.
I want to add a cautionary note that I don’t believe we are ready to drop anthracyclines without a prospective trial. We have decades of efficacy data with anthracyclines, so although I love the TC regimen, for patients with node-positive disease I believe that one of the proven three-or four-drug regimens — TAC, dose-dense AC/paclitaxel or FEC followed by docetaxel — is still the standard.
![]() DR LOVE: What about patients with node-negative disease in the adjuvant
setting?
DR LOVE: What about patients with node-negative disease in the adjuvant
setting?
![]() DR O’SHAUGHNESSY: At ASCO 2008, Miguel Martin presented the five-year
efficacy analysis of the GEICAM 9805 trial, which showed that adjuvant TAC
was associated with a significant improvement in disease-free survival compared
to FAC in patients with high-risk, node-negative breast cancer (Martin 2008).
DR O’SHAUGHNESSY: At ASCO 2008, Miguel Martin presented the five-year
efficacy analysis of the GEICAM 9805 trial, which showed that adjuvant TAC
was associated with a significant improvement in disease-free survival compared
to FAC in patients with high-risk, node-negative breast cancer (Martin 2008).
I believe that we should treat patients with node-negative disease who will benefit from chemotherapy, such as those with ER-negative disease or highly proliferative ER-positive disease, with effective chemotherapy. At MD Anderson, all patients who receive adjuvant chemotherapy receive 12 doses of weekly paclitaxel followed by four cycles of FAC.
I believe that patients who we feel will benefit significantly from chemotherapy should receive an anthracycline-based regimen such as TAC or dose-dense chemotherapy or the MD Anderson regimen. Also, these patients at high risk are eligible for our TC versus TAC trial.
However, for patients who have more indolent disease, I believe a role exists for the four cycles of TC in patients whose benefit from chemotherapy may be small — somewhere between zero and three percent.
Track 13
![]() DR LOVE: Oncotype DX is now reporting quantitative ER and PR in
addition to a Recurrence Score. Do you see that being helpful in practice?
DR LOVE: Oncotype DX is now reporting quantitative ER and PR in
addition to a Recurrence Score. Do you see that being helpful in practice?
![]() DR O’SHAUGHNESSY: Yes, it is helpful because with the RT-PCR mRNA
methodology, you have approximately a 200-fold or higher dynamic range of
ER and PR. The way we currently test ER, for example, results cluster at zero
or maybe 10 to 30 percent, with a few at 50 and some at 90 or 100 percent,
and the PR antibody is unreliable.
DR O’SHAUGHNESSY: Yes, it is helpful because with the RT-PCR mRNA
methodology, you have approximately a 200-fold or higher dynamic range of
ER and PR. The way we currently test ER, for example, results cluster at zero
or maybe 10 to 30 percent, with a few at 50 and some at 90 or 100 percent,
and the PR antibody is unreliable.
![]() DR LOVE: How does this quantitative information help you clinically?
DR LOVE: How does this quantitative information help you clinically?
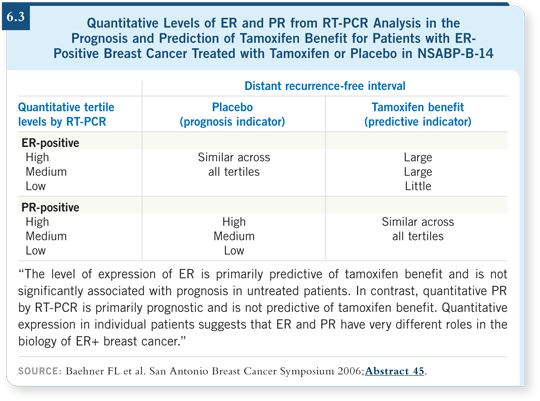
![]() DR O’SHAUGHNESSY: The quantitative data provide more information on the extent to which women will benefit from endocrine therapy. At the San
Antonio Breast Cancer Symposium in 2006, data were presented on quantitative
ER and PR from the NSABP-B-14 trial, comparing tamoxifen to placebo, and
they categorized it by tertiles (Baehner 2006). They definitively demonstrated
that the degree to which a patient will benefit from tamoxifen is dependent on
the ER tertile — the stronger the ER, the greater the benefit.
DR O’SHAUGHNESSY: The quantitative data provide more information on the extent to which women will benefit from endocrine therapy. At the San
Antonio Breast Cancer Symposium in 2006, data were presented on quantitative
ER and PR from the NSABP-B-14 trial, comparing tamoxifen to placebo, and
they categorized it by tertiles (Baehner 2006). They definitively demonstrated
that the degree to which a patient will benefit from tamoxifen is dependent on
the ER tertile — the stronger the ER, the greater the benefit.
It is interesting that ER was not prognostic and, inversely, PR was prognostic but not predictive of benefit (6.3).
EDITOR
Neil Love, MD
INTERVIEWS
Nancy E Davidson, MD
- Select publications
Professor John Crown, MD
- Select publications
Kathy D Miller, MD
- Select publications
Peter M Ravdin, MD, PhD
- Select publications
THREE PERSPECTIVES ON US COOPERATIVE GROUP RESEARCH
Norman Wolmark, MD
- Select publications
Joyce O’Shaughnessy, MD
- Select publications
Eric P Winer, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity