
 |
||||||||

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 2, 4
![]() DR LOVE: What’s your view on the role of anthracyclines in the adjuvant
setting?
DR LOVE: What’s your view on the role of anthracyclines in the adjuvant
setting?
![]() PROF CROWN: We know that topoisomerase II (TOPO II) is one of the
principal targets for the anthracyclines. Dr Slamon’s data from BCIRG 005
strongly suggest that HER2-negative tumors are invariably TOPO II-negative
(Slamon 2007). In addition, the recent meta-analysis from Gennari strongly
suggests that the benefit — a fairly weak benefit — we have observed in the past from anthracycline-containing versus nonanthracycline-containing
regimens in the adjuvant setting may be confined to the HER2-positive
population (Gennari 2008). Based on the combined data, we would hypothesize
further that it is confined to the TOPO II-positive subset.
PROF CROWN: We know that topoisomerase II (TOPO II) is one of the
principal targets for the anthracyclines. Dr Slamon’s data from BCIRG 005
strongly suggest that HER2-negative tumors are invariably TOPO II-negative
(Slamon 2007). In addition, the recent meta-analysis from Gennari strongly
suggests that the benefit — a fairly weak benefit — we have observed in the past from anthracycline-containing versus nonanthracycline-containing
regimens in the adjuvant setting may be confined to the HER2-positive
population (Gennari 2008). Based on the combined data, we would hypothesize
further that it is confined to the TOPO II-positive subset.
![]() DR LOVE: In terms of nonanthracycline options, what’s your take on the
US Oncology data on TC (docetaxel/cyclophosphamide)?
DR LOVE: In terms of nonanthracycline options, what’s your take on the
US Oncology data on TC (docetaxel/cyclophosphamide)?
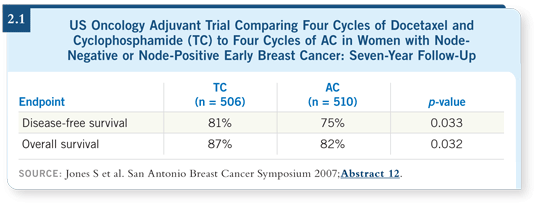
![]() PROF CROWN: The TC regimen is receiving a good deal of attention, and the
TC versus AC trial was an excellent study (Jones 2006; [2.1]). In my practice,
I use the TC regimen frequently and have largely moved to a nonanthracycline
regimen as my standard for these patients with HER2-negative tumors.
PROF CROWN: The TC regimen is receiving a good deal of attention, and the
TC versus AC trial was an excellent study (Jones 2006; [2.1]). In my practice,
I use the TC regimen frequently and have largely moved to a nonanthracycline
regimen as my standard for these patients with HER2-negative tumors.
My decision was based on a number of factors, including the repeated observation that for patients with HER2-negative disease, it’s difficult to know exactly how much benefit they are receiving. In addition, the toxicity associated with anthracyclines may be worse than we thought, including an alarming report detailing as much as a one percent incidence of leukemia and myelodysplastic syndrome among patients treated with aggressive anthracycline regimens. In the Irish Clinical Oncology Research Group, we have launched a new generation of studies for our patients with HER2-negative early breast cancer. Soon we will be enrolling patients on a large-scale adjuvant pilot trial of TC with bevacizumab, and I know others are piloting similar regimens.
![]() DR LOVE: My understanding is that the NSABP and US Oncology are
expanding the TC-TAC trial comparing TC to TAC to a larger study with a
third arm also evaluating TC/bevacizumab.
DR LOVE: My understanding is that the NSABP and US Oncology are
expanding the TC-TAC trial comparing TC to TAC to a larger study with a
third arm also evaluating TC/bevacizumab.
![]() PROF CROWN: I’d be supportive of that trial. I’m eager to know the answers to
both the anthracycline-versus-no-anthracycline and the bevacizumab questions.
PROF CROWN: I’d be supportive of that trial. I’m eager to know the answers to
both the anthracycline-versus-no-anthracycline and the bevacizumab questions.
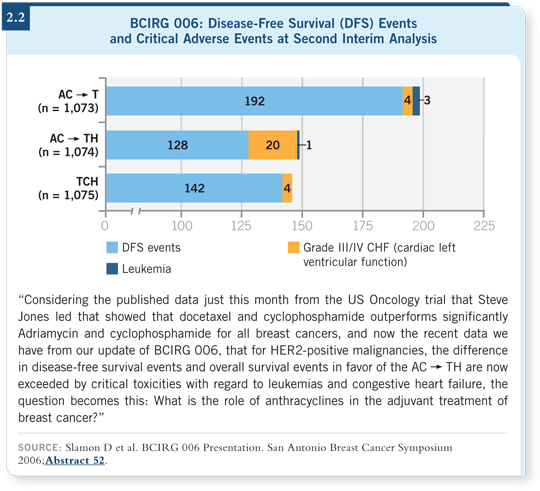
![]() DR LOVE: In terms of the HER2-positive population, based on the BCIRG
006 data, the TCH regimen appears to have similar efficacy to anthracycline-based
therapy and less cardiotoxicity (2.2). What do you think of those data,
and how are you treating your patients?
DR LOVE: In terms of the HER2-positive population, based on the BCIRG
006 data, the TCH regimen appears to have similar efficacy to anthracycline-based
therapy and less cardiotoxicity (2.2). What do you think of those data,
and how are you treating your patients?
![]() PROF CROWN: I chaired that study with Dr Slamon, so I may not be the most
unbiased observer, but I have stopped administering regimens containing both
trastuzumab and an anthracycline. I routinely administer TCH as my adjuvant
regimen for HER2-positive disease.
PROF CROWN: I chaired that study with Dr Slamon, so I may not be the most
unbiased observer, but I have stopped administering regimens containing both
trastuzumab and an anthracycline. I routinely administer TCH as my adjuvant
regimen for HER2-positive disease.
Track 8
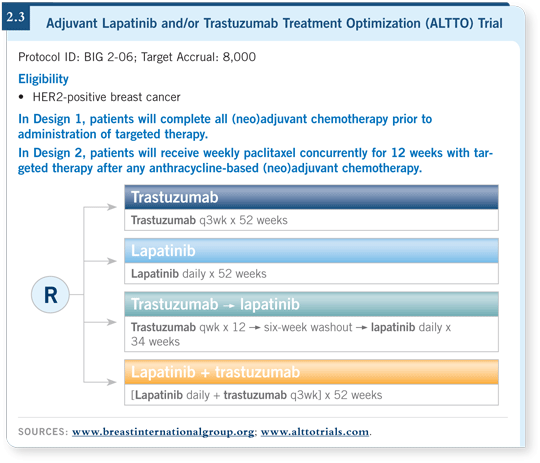
![]() DR LOVE: The ALTTO adjuvant trial (2.3) is evaluating lapatinib and
the combination of lapatinib and trastuzumab. What do we know about
the mechanism of action of lapatinib and the effect of combining it with
trastuzumab?
DR LOVE: The ALTTO adjuvant trial (2.3) is evaluating lapatinib and
the combination of lapatinib and trastuzumab. What do we know about
the mechanism of action of lapatinib and the effect of combining it with
trastuzumab?
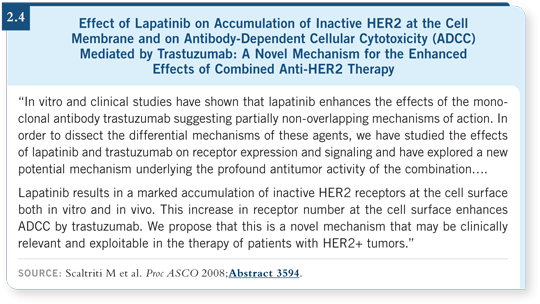
![]() PROF CROWN: Lapatinib is a fascinating agent, and what interests me most is
the possibility that combined lapatinib and trastuzumab therapy may produce
superior results compared to either molecularly targeted agent alone. Several
lab groups have shown an additive value or even a synergy between these two
agents in HER2-positive cell lines. In addition, a trial presented at ASCO 2008
by Dr O’Shaughnessy suggested that this may be applicable in the clinic, which
is good news for those interested in adjuvant trials combining these agents
(Scaltriti 2008; [2.4]).
PROF CROWN: Lapatinib is a fascinating agent, and what interests me most is
the possibility that combined lapatinib and trastuzumab therapy may produce
superior results compared to either molecularly targeted agent alone. Several
lab groups have shown an additive value or even a synergy between these two
agents in HER2-positive cell lines. In addition, a trial presented at ASCO 2008
by Dr O’Shaughnessy suggested that this may be applicable in the clinic, which
is good news for those interested in adjuvant trials combining these agents
(Scaltriti 2008; [2.4]).
At this point, lapatinib offers heavily treated patients who experience disease progression despite trastuzumab another treatment with the prospect of further meaningful benefit, which is always worthwhile in the palliative setting. However, my hope is that in combining it with trastuzumab, we will see even better results.
The Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization (ALTTO) trial is being inaugurated, and we hope a broader portfolio of studies evaluating the combination will become available.
![]() DR LOVE: What do we know about lapatinib in HER2-negative disease?
DR LOVE: What do we know about lapatinib in HER2-negative disease?
![]() PROF CROWN: The benefit of lapatinib appears to be confined to HER2-
positive breast cancer. If activity occurs in HER2-negative disease, it is minimal.
This may seem surprising as four or five years ago lapatinib was being initiated
into trials because of its dual action on EGFR and HER2. Although an interplay
may occur between these two receptors that accounts for some of lapatinib’s
activity, using it to target HER2-negative tumors by virtue of a targeting effect
on EGFR does not appear to be a productive strategy at the moment.
PROF CROWN: The benefit of lapatinib appears to be confined to HER2-
positive breast cancer. If activity occurs in HER2-negative disease, it is minimal.
This may seem surprising as four or five years ago lapatinib was being initiated
into trials because of its dual action on EGFR and HER2. Although an interplay
may occur between these two receptors that accounts for some of lapatinib’s
activity, using it to target HER2-negative tumors by virtue of a targeting effect
on EGFR does not appear to be a productive strategy at the moment.
![]() DR LOVE: In HER2-positive cancer, what do we know about the contribution,
if any, of the anti-EGFR effect of lapatinib?
DR LOVE: In HER2-positive cancer, what do we know about the contribution,
if any, of the anti-EGFR effect of lapatinib?
![]() PROF CROWN: Lapatinib works in a fundamentally different way than
trastuzumab. It works on the intracellular side, and a number of different
downstream regulators of HER2 may be differentially affected by lapatinib as
opposed to trastuzumab.
PROF CROWN: Lapatinib works in a fundamentally different way than
trastuzumab. It works on the intracellular side, and a number of different
downstream regulators of HER2 may be differentially affected by lapatinib as
opposed to trastuzumab.
Track 9
![]() DR LOVE: You recently published a paper on the important issue of
lapatinib-induced diarrhea (Crown 2008). What side effects do you
generally see with this agent?
DR LOVE: You recently published a paper on the important issue of
lapatinib-induced diarrhea (Crown 2008). What side effects do you
generally see with this agent?
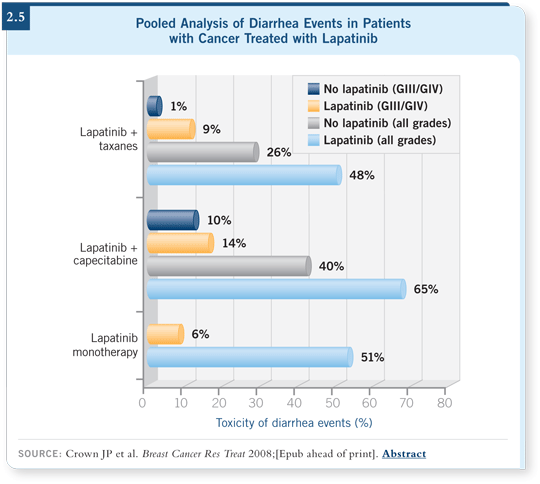
![]() PROF CROWN: In general, lapatinib is a well-tolerated drug. A mild level of
skin rash can occur, and a little diarrhea is relatively common (2.5). Severe
diarrhea is not common, and when it does occur, it must be managed aggressively.
The combination of capecitabine and lapatinib can cause diarrhea, but
in the absence of a diarrhea-inducing chemotherapy agent, diarrhea is less of a
problem.
PROF CROWN: In general, lapatinib is a well-tolerated drug. A mild level of
skin rash can occur, and a little diarrhea is relatively common (2.5). Severe
diarrhea is not common, and when it does occur, it must be managed aggressively.
The combination of capecitabine and lapatinib can cause diarrhea, but
in the absence of a diarrhea-inducing chemotherapy agent, diarrhea is less of a
problem.
In my jurisdiction in Ireland, lapatinib use is confined to a specific indication, which is coadministration with capecitabine to patients with HER2-positive metastatic breast cancer whose disease has progressed after anthracyclines, taxanes and trastuzumab. These patients have been heavily pretreated and have particularly bad cancer, so they need to be treated carefully.
Patients need to be attuned to the possibility of side effects, and I warn them about diarrhea. If they experience severe diarrhea, we advise them to stop taking the tablets and call us. Depending on where they are in their capecitabine cycle, we make recommendations on dose reduction, generally of the capecitabine, and we administer antidiarrhea therapy as needed.
![]() DR LOVE: How do you manage the rash?
DR LOVE: How do you manage the rash?
![]() PROF CROWN: As with any EGFR rash, we generally stop the treatment for a
few days to let it settle down. For many patients it’s simply a matter of reinstituting
the drug at a lower dose, although some need specific interventions
such as antibiotics.
PROF CROWN: As with any EGFR rash, we generally stop the treatment for a
few days to let it settle down. For many patients it’s simply a matter of reinstituting
the drug at a lower dose, although some need specific interventions
such as antibiotics.
EDITOR
Neil Love, MD
INTERVIEWS
Nancy E Davidson, MD
- Select publications
Professor John Crown, MD
- Select publications
Kathy D Miller, MD
- Select publications
Peter M Ravdin, MD, PhD
- Select publications
THREE PERSPECTIVES ON US COOPERATIVE GROUP RESEARCH
Norman Wolmark, MD
- Select publications
Joyce O’Shaughnessy, MD
- Select publications
Eric P Winer, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity