
 |
||||||||

| Tracks 1-12 | ||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: What is your take on the ABCSG-12 data presented at the
ASCO plenary session?
DR LOVE: What is your take on the ABCSG-12 data presented at the
ASCO plenary session?
![]() DR DAVIDSON: This exciting Austrian trial addressed two questions about
premenopausal patients with estrogen receptor (ER)-positive early breast
cancer (Gnant 2008). First, it evaluated endocrine therapy, randomly assigning
patients receiving an LHRH agonist to either tamoxifen or anastrozole, and
no significant efficacy advantage was evident for either arm.
DR DAVIDSON: This exciting Austrian trial addressed two questions about
premenopausal patients with estrogen receptor (ER)-positive early breast
cancer (Gnant 2008). First, it evaluated endocrine therapy, randomly assigning
patients receiving an LHRH agonist to either tamoxifen or anastrozole, and
no significant efficacy advantage was evident for either arm.
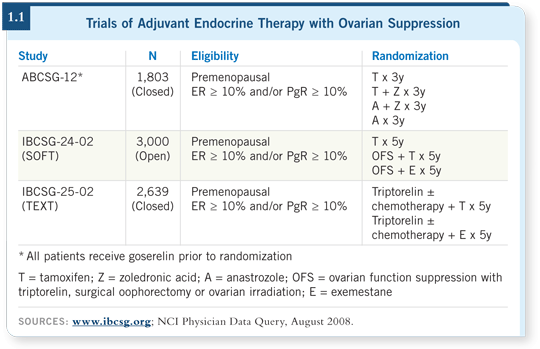
These data tell me that the international trials SOFT and TEXT are absolutely vital to determine optimal endocrine therapy (1.1) for these women. Many people expected that the aromatase inhibitor combination would be superior, and I hope they will now step back and realize that this question is still unanswered.
Another interesting point regarding these data is that the vast majority of patients in the study had not received chemotherapy. Nonetheless, the outcome for these women was extremely good — approximately six percent experienced recurrence in the first five years.
This should reassure us that young women don’t always need chemotherapy. We should be guided much more by the biology of the tumor and less by our bias that perhaps youth means the patient needs to receive chemotherapy.
Tracks 3-4
![]() DR LOVE: What are your thoughts about the second randomization in
ABCSG-12 to zoledronic acid (ZDA) versus no bisphosphonate therapy
in these premenopausal patients, demonstrating about 35 percent fewer
events in patients receiving ZDA?
DR LOVE: What are your thoughts about the second randomization in
ABCSG-12 to zoledronic acid (ZDA) versus no bisphosphonate therapy
in these premenopausal patients, demonstrating about 35 percent fewer
events in patients receiving ZDA?
![]() DR DAVIDSON: That randomization was based on data from previous clodronate
trials that suggested a bisphosphonate might have antineoplastic effects in
addition to bone-preserving effects. That would certainly be desirable when
treating young women with endocrine therapy.
DR DAVIDSON: That randomization was based on data from previous clodronate
trials that suggested a bisphosphonate might have antineoplastic effects in
addition to bone-preserving effects. That would certainly be desirable when
treating young women with endocrine therapy.
The data presented at ASCO indeed demonstrated that the patients who received zoledronic acid had longer disease-free survival. They experienced fewer recurrences, and not only bone recurrences as one might have predicted, but fewer recurrences at other sites also (4.2, page 24).
![]() DR LOVE: As a result of this study, will you be recommending adjuvant
bisphosphonates for this subset of patients in your clinical practice?
DR LOVE: As a result of this study, will you be recommending adjuvant
bisphosphonates for this subset of patients in your clinical practice?
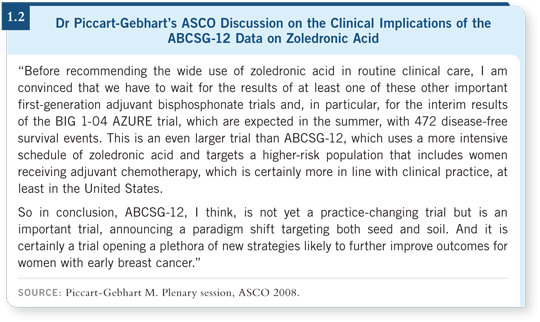
![]() DR DAVIDSON: I was affected by Martine Piccart-Gebhart’s discussion of this abstract (1.2). She decided that before she would embrace routine adjuvant
bisphosphonate therapy in her practice, she would wait to see the outcome of
other large randomized bisphosphonate trials that are coming to an end.
DR DAVIDSON: I was affected by Martine Piccart-Gebhart’s discussion of this abstract (1.2). She decided that before she would embrace routine adjuvant
bisphosphonate therapy in her practice, she would wait to see the outcome of
other large randomized bisphosphonate trials that are coming to an end.
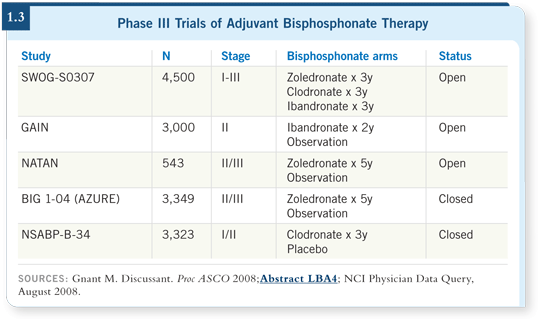
She had a slide illustrating that several of these studies are in progress, incorporating thousands of women, and she suggested that if we’re lucky, data might be released from the first of these later this year (1.3).
I took her counsel. ABCSG-12 is a relatively small trial, and of the three randomized clodronate trials that have been conducted, although two appeared positive, the third was negative. At least for the moment, I am not using zoledronic acid routinely for premenopausal women, but I am waiting for the data from the ongoing trials.
Track 5
![]() DR LOVE: Can you discuss the rationale for the prospective study at Johns
Hopkins examining adjuvant anastrozole and gene promoter methylation
in the contralateral breast of women at high risk for breast cancer (Prowell
2008)?
DR LOVE: Can you discuss the rationale for the prospective study at Johns
Hopkins examining adjuvant anastrozole and gene promoter methylation
in the contralateral breast of women at high risk for breast cancer (Prowell
2008)?
![]() DR DAVIDSON: My colleague Vered Stearns has been enthusiastic about the
idea of using the contralateral breast as a short-term model system to evaluate
chemoprevention.
DR DAVIDSON: My colleague Vered Stearns has been enthusiastic about the
idea of using the contralateral breast as a short-term model system to evaluate
chemoprevention.
She is also interested in examining molecular markers of response, both in the tumor and in the patient. The questions addressed in this study were whether that could be a useful way to monitor response to aromatase inhibitors and whether these might be useful in the prevention setting.
The study consists of postmenopausal women who were about to receive adjuvant anastrozole and were asked to do so in the context of a trial that allowed us to collect a lot of data. Patients were required to undergo a biopsy of the contralateral breast before they were exposed to the aromatase inhibitor and again after six months of exposure.
The first report consisted of 25 women, and we examined the status of approximately a dozen genes that are frequently methylated in breast cancer. We found that methylation of one or more of these genes was observed in more than 80 percent of these women who were at high risk of breast cancer in the contralateral breast. In approximately half of them, we observed a decrease in the quantitative methylation index after six months of anastrozole therapy.
Tracks 7-9
![]() DR LOVE: You have been very involved in clinical trials, such as MA17,
trying to determine the optimal duration of adjuvant endocrine therapy.
What is your current approach to this decision outside a trial setting?
DR LOVE: You have been very involved in clinical trials, such as MA17,
trying to determine the optimal duration of adjuvant endocrine therapy.
What is your current approach to this decision outside a trial setting?
![]() DR DAVIDSON: Increasingly, I am a believer in long-term strategies for
hormone-responsive breast cancer. Many of us were struck more than a
decade ago by the ECOG study published by Saphner, Tormey and Gray that
examined the natural history of ER-positive versus ER-negative breast cancer
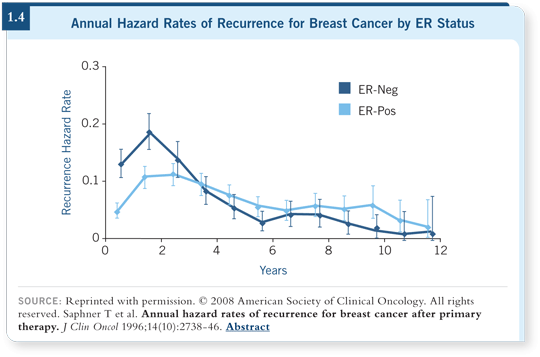
and suggested a difference (Saphner 1996; [1.4]). We appreciate that difference
more now, and we have more options, such as aromatase inhibitors, for
addressing this issue.
DR DAVIDSON: Increasingly, I am a believer in long-term strategies for
hormone-responsive breast cancer. Many of us were struck more than a
decade ago by the ECOG study published by Saphner, Tormey and Gray that
examined the natural history of ER-positive versus ER-negative breast cancer
and suggested a difference (Saphner 1996; [1.4]). We appreciate that difference
more now, and we have more options, such as aromatase inhibitors, for
addressing this issue.
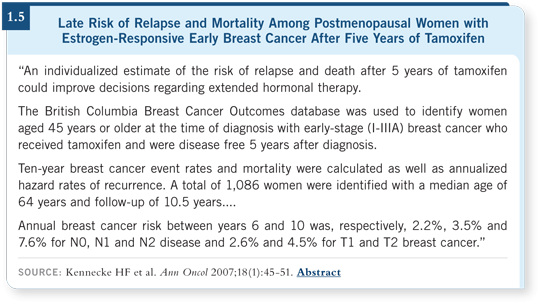
However, I’m not sure how long “long-term” is, and I wish we had a better way to stratify a patient’s risk. When a patient who had a node-negative, 1-cm, ER-positive breast tumor comes to the end of five years of tamoxifen or five years of an aromatase inhibitor, I wonder whether she needed all that therapy and whether some of them need more (1.5).
The question of how long to administer these therapies is a huge issue, and it’s difficult to address in trials because these studies require such long follow-up (1.5). I am hoping that some of the biomarker research will help us hone that down over time.
![]() DR LOVE: Let’s get more specific. How would you approach a premenopausal
patient who has positive nodes, has received five years of tamoxifen and is
tolerating it well?
DR LOVE: Let’s get more specific. How would you approach a premenopausal
patient who has positive nodes, has received five years of tamoxifen and is
tolerating it well?
![]() DR DAVIDSON: I discuss the information that exists and present three strategies.
One is continuing tamoxifen, year to year, and I tell the patient that
although I’m certain it contributes to toxicity, I’m not quite sure what it contributes in terms of benefit. A second option is to quit therapy altogether,
and the third is to transition to an aromatase inhibitor with an LHRH agonist.
I’ve had patients select each of these options. Most commonly they stop
therapy because they feel they are done and ready to move on. Rarely, the
patients elect to cross over to the hormonal blockade. The few patients who do
opt to continue tamoxifen are often women who perceive themselves to be at
high risk.
DR DAVIDSON: I discuss the information that exists and present three strategies.
One is continuing tamoxifen, year to year, and I tell the patient that
although I’m certain it contributes to toxicity, I’m not quite sure what it contributes in terms of benefit. A second option is to quit therapy altogether,
and the third is to transition to an aromatase inhibitor with an LHRH agonist.
I’ve had patients select each of these options. Most commonly they stop
therapy because they feel they are done and ready to move on. Rarely, the
patients elect to cross over to the hormonal blockade. The few patients who do
opt to continue tamoxifen are often women who perceive themselves to be at
high risk.
![]() DR LOVE: When you see a premenopausal patient who experienced chemotherapy-induced amenorrhea and has recently completed five years of tamoxifen,
do you use an aromatase inhibitor?
DR LOVE: When you see a premenopausal patient who experienced chemotherapy-induced amenorrhea and has recently completed five years of tamoxifen,
do you use an aromatase inhibitor?
![]() DR DAVIDSON: I believe all of us were captivated by Ian Smith’s paper
examining these patients who then received an aromatase inhibitor (Smith
2006). In some cases they retained or regained ovarian function, and one even
became pregnant. In each case, we think carefully about whether we believe
the benefit outweighs the risk.
DR DAVIDSON: I believe all of us were captivated by Ian Smith’s paper
examining these patients who then received an aromatase inhibitor (Smith
2006). In some cases they retained or regained ovarian function, and one even
became pregnant. In each case, we think carefully about whether we believe
the benefit outweighs the risk.
In my practice, I discuss with the patient whether she wants to consider extended endocrine therapy. If she does, then I stop the tamoxifen for a few months to see how she feels off therapy, establish a new baseline and watch to see whether her ovarian function kicks in, either clinically or by laboratory parameters. If the patient then begins an aromatase inhibitor, I watch her carefully. I’ve been following these patients clinically, although I’ve considered monitoring laboratory studies too. The key there, of course, is to have access to a lab that can perform high-sensitivity estrogen assays.
Track 10
![]() DR LOVE: What’s your take on the pathophysiology of arthralgias
secondary to aromatase inhibitors?
DR LOVE: What’s your take on the pathophysiology of arthralgias
secondary to aromatase inhibitors?
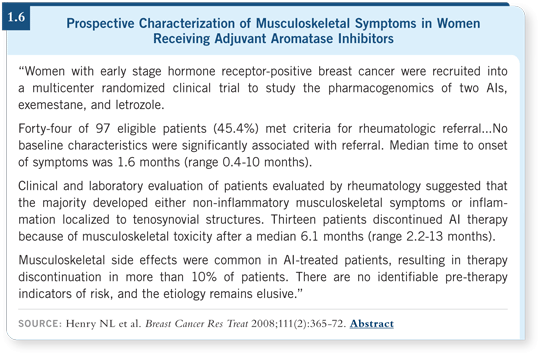
![]() DR DAVIDSON: At Hopkins we are involved in a randomized clinical trial
to study the pharmacogenomics of exemestane and letrozole in women with
early-stage, hormone receptor-positive breast cancer. Lynn Henry published a
paper examining the first 100 patients in the trial, and a large proportion saw
a rheumatologist because they crossed a predefined symptomatology threshold
(Henry 2008; [1.6]).
DR DAVIDSON: At Hopkins we are involved in a randomized clinical trial
to study the pharmacogenomics of exemestane and letrozole in women with
early-stage, hormone receptor-positive breast cancer. Lynn Henry published a
paper examining the first 100 patients in the trial, and a large proportion saw
a rheumatologist because they crossed a predefined symptomatology threshold
(Henry 2008; [1.6]).
The findings are all over the map, and no single explanation for these symptoms is clear to me. What to do about them is also complicated, and one purpose of our study is to determine whether it is possible to predict which aromatase inhibitor a patient will tolerate better or perhaps to identify patients who are more prone to these musculoskeletal symptoms.
I believe these symptoms were underreported in the large, randomized aromatase inhibitor trials. Now that we have more experience and are paying attention to this side effect, we are recognizing that the problem is critical to address because compliance is important with these drugs, and if women aren’t feeling well, they may not be as compliant.
EDITOR
Neil Love, MD
INTERVIEWS
Nancy E Davidson, MD
- Select publications
Professor John Crown, MD
- Select publications
Kathy D Miller, MD
- Select publications
Peter M Ravdin, MD, PhD
- Select publications
THREE PERSPECTIVES ON US COOPERATIVE GROUP RESEARCH
Norman Wolmark, MD
- Select publications
Joyce O’Shaughnessy, MD
- Select publications
Eric P Winer, MD
- Select publications
Breast Cancer Update:
A CME Audio Series and Activity