| You are here: Home: BCU 4|2002: Program Supplement: Treatment of metastic disease
| Treatment
of the Premenopausal Patient with
ER-positive, HER2-negative Metastatic Disease |
|
| What therapy would you utilize in a
43-year-old woman with asymptomatic bone metastases
and ER-positive, HER2-negative disease?
|
|
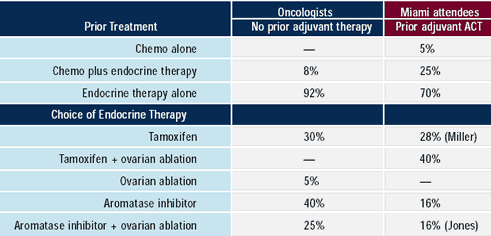
Almost all oncologists would give endocrine therapy to asymptomatic,
ER-positive, premenopausal patients with bone metastases —
primarily tamoxifen alone or combined with ovarian ablation/suppression
or an aromatase inhibitor combined with ovarian ablation / suppression.
It is noteworthy that 16-40% of oncologists would use single-agent
aromatase inhibitors in premenopausal women, while 16-25% would
use it in conjunction with ovarian ablation/suppression. A romatase
inhibitors are not indicated in the treatment of premenopausal women.
Aebi S et al. Is chemotherapy alone adequate for young women
with oestrogen receptor-positive breast cancer? Lancet 2000;355(9218):1869-74.
Abstract
Buzdar AU. Endocrine therapy in the treatment of metastatic
breast cancer. Semin Oncol 2001;28(3):291-304. Abstract
Cheung KLet al. The combined use of goserelin and anastrozole
as second-line endocrine therapy in premenopausal women with advanced
breast cancer - a study of its clinical and endocrine effects.
Proc ASCO 2001; Abstract
1937.
Forward D et al. Combined use of goserelin (Zoladex) and anastrozole
(Arimidex) in premenopausal women with metastatic breast cancer
(MBC). Proc ASCO 2000; Abstract
582.
Hoffken K, Kath R. The role of LH-RH analogues in the adjuvant
and palliative treatment of breast cancer. Recent Results
Cancer Res 2000;153:61-70. Abstract
| Treatment
of the Premenopausal Patient with
ER-positive, HER2-negative Metastatic Disease |
|
| What therapy would you utilize in a
43-year-old, very ill woman with visceral metastases
and ER-positive, HER2-negative disease?
|
|
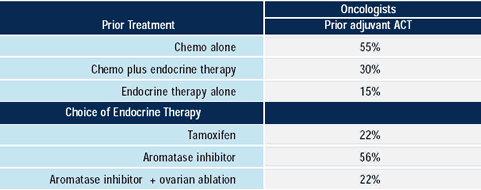
In very ill women with visceral metastases, 80% of oncologists
would utilize chemotherapy alone or combined with endocrine therapy.
Whether or not endocrine therapy alone is adequate in an asymptomatic
patient with visceral metastases is a topic of controversy. Research
leaders often note that endocrine therapy usually takes somewhat
longer than chemotherapy to elicit a tumor response, so that clinical
judgment involves assessing whether the patient is stable enough
to wait for a response to endocrine treatment. In the adjuvant setting,
chemotherapy and endocrine therapy have been proven to have an additive
or synergistic effect.
Ingle JN et al. Evaluation of tamoxifen plus letrozole with
assessment of pharmacokinetic interaction in postmenopausal women
with metastatic breast cancer. Clin Cancer Res 1999;5(7):1642-9.
Abstract
Jordan C. Historical perspective on hormonal therapy of advanced
breast cancer. Clin Ther 2002; 24 Suppl A:A3-A16. Abstract
Klijn JG et al. Combined tamoxifen and luteinizing hormone-releasing
hormone (LHRH) agonist versus LHRH agonist alone in premenopausal
advanced breast cancer: A meta-analysis of four randomized trials.
J Clin Oncol 2001;19(2):343-53. Abstract
Michaud LB et al. Combination endocrine therapy in the management
of breast cancer. Oncologist 2001;6(6):538-46. Abstract
| Treatment
of Premenopausal Patients with
ER-negative, HER2-negative Metastatic Disease |
|
| What therapy would you utilize in the
following 43-year-old women with ER-negative, HER2-negative
metastatic breast cancer, who received prior adjuvant
ACT?
|
|
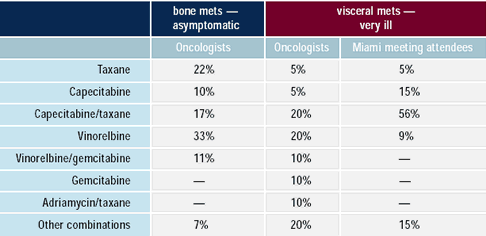
With an increasing fraction of breast cancer patients receiving
taxane-based adjuvant systemic therapy, the dilemma of the patient
presenting shortly t h e reafter with metastatic disease is becoming
more common. There is considerable variation in choice of chemotherapy
regimen in this situation. About one year after the initial results
of the capecitabine/docetaxel study were first reported — demonstrating
a survival advantage and a relatively favorable side - effect profile
— more than half of attendees to the Miami meeting would choose
this regimen, but relatively few physicians in practice follow this
approach.
Crown J. Nonanthracycline-containing docetaxel-based combinations
in metastatic breast cancer. Oncologist 2001;6 Suppl
3:17-21. Abstract
Domenech GH, Vogel CL. A review of vinorelbine in the treatment
of breast cancer. Clin Breast Cancer 2001 Jul;2(2):113-28.
Abstract
Miles D. Survival benefit with Xeloda (capecitabine)/docetaxel
vs docetaxel: Analysis of post-study therapy. Breast Cancer
Res Treat 2001; Abstract
442.
Seidman AD. The evolving role of gemcitabine in the management
of breast cancer. Oncology 2001;60(3):189-98. Abstract
Vukelja SJ et al. Xeloda (capecitabine) plus docetaxel combination
therapy in locally advanced/metastatic breast cancer: Latest results.
Breast Cancer Res Treat 2001; Abstract
352.
| Treatment
of Postmenopausal Patients with
ER-negative, HER2-negative Metastatic Disease |
|
| What therapy would you utilize in the
following 63-year-old women with ER-negative, HER2-negative
metastatic breast cancer, who received prior adjuvant
ACT?
|
|
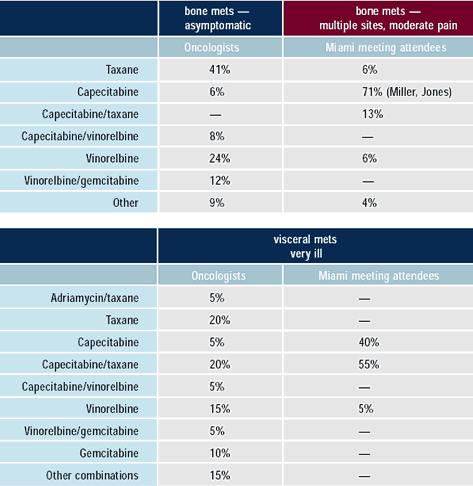
The older patient relapsing after taxane-based adjuvant systemic
therapy is commonly treated with capecitabine, either alone or in
combination with a taxane. Over one-half of the oncologists attending
the 2002 Miami Breast Cancer Conference would treat a 63 - year-old
woman with visceral metastases with the combination of capecitabine
/ docetaxel. Another agent commonly utilized in this situation is
vinorelbine.
Maher JF, Villalona-Calero MA. Taxanes and capecitabine in combination:
Rationale and clinical results. Clin Breast Cancer 2002;2(4):287-93.
Abstract
| Single-Agent
Versus Combination
Chemotherapy in Metastatic Disease |
|
| Oncologists: Would you utilize single-agent
or combination chemotherapy in the following women with
ER-negative, HER2-negative metastatic breast cancer
who received prior adjuvant ACT?
|
|
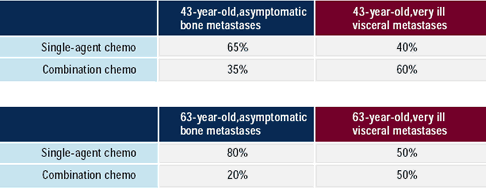
Proponents of sequential single-agent chemotherapy in metastatic
breast cancer believe there is no survival advantage for combination
chemotherapy, and that patients are exposed to less toxicity with
single agents.
Oncologists are more likely to use combination chemotherapy for
patients with rapidly progressing, visceral metastases in whom a
rapid response to therapy is critical. Since earlier and more effective
disease control may translate into a better quality of life, some
breast cancer research leaders use combination regimens earlier
in the metastatic setting. If clinical trial results with newer,
rationally derived, synergistic and relatively less toxic combinations
demonstrate progression - free and overall survival advantages,
more oncologists may adopt these combinations. Most recently, a
survival advantage was observed for capecitabine /docetaxel in a
trial comparing it to docetaxel alone.
In this survey, three-quarters of oncologists reported that 60%
or more of their patients receive combination chemotherapy at some
point in the treatment of their metastatic disease.
Sledge GW Jr et al. Phase III trial of doxorubicin versus paclitaxel
versus doxorubicin plus paclitaxel as first-line therapy for metastatic
breast cancer: An Intergroup trial. Proc ASCO 1997; Abstract
2.
| Treatment
of Postmenopausal Patients with
ER-positive, HER2-negative Metastatic Disease |
|
| Miami meeting attendees: What
is your usual first-line hormonal agent for a 65-year-old
breast cancer patient with ER-positive, HER2-negative
bone metastases, who has not received adjuvant endocrine
therapy?
Oncologists: What therapy would you utilize
in the following 63-year-old, ER-positive, HER2-negative
patients with metastatic disease who received prior
adjuvant ACT?
|
|
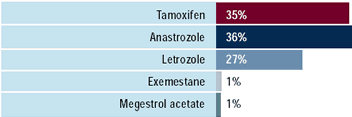
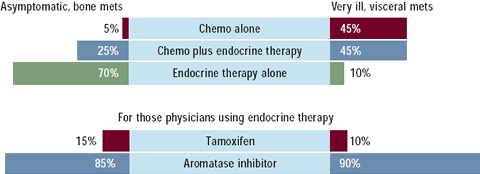
A number of clinical trials have demonstrated improved efficacy
and less toxicity with the third-generation aromatase inhibitors
compared to tamoxifen as first-line therapy for metastatic breast
cancer. Currently, two-thirds of oncologists use a romatase inhibitors
as first-line therapy with anastrozole and letrozole utilized about
equally. About half of oncologists use a combination of chemotherapy
and hormonal therapy in the highly symptomatic patient with visceral
disease.
Mouridsen H et al. Superior efficacy of letrozole versus tamoxifen
as first-line therapy for postmenopausal women with advanced breast
cancer: Results of a phase III study of the International Letrozole
Breast Cancer Group. J Clin Oncol 2001;19(10):2596-606.
Abstract
Nabholtz JM et al. Anastrozole is superior to tamoxifen as
first-line therapy for advanced breast cancer in postmenopausal
women: Results of a North American multicenter randomized trial.
Arimidex Study Group. J Clin Oncol 2000;18(22):3758-67.
Abstract
| Treatment
of the Elderly Patient with Metastatic Breast Cancer |
|
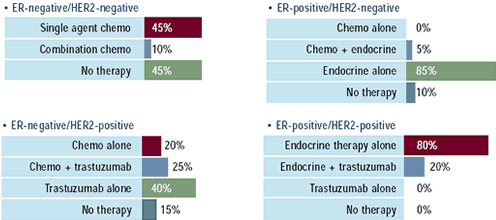
| Oncologists: How would you treat
the following 78-year-old women with asymptomatic bone
metastases, who received no prior adjuvant therapy?
|
|
 |
 |
Oncologists are reluctant to expose elderly patients to the morbidity
associated with chemotherapy. Depending on the ER and HER2 status,
5-55% of oncologists would treat a 78-year-old woman with chemotherapy,
most often employing single-agent therapy. In ER-positive patients
90-100% of physicians would utilize endocrine therapy, and nearly
all would use an aromatase inhibitor. In ER-negative, HER2-positive
patients, nearly two-thirds of oncologists would use trastuzumab,
alone or in combination with chemotherapy; however, they were still
more likely to use endocrine therapy in the ER-positive, HER2-positive
patient. Research leaders believe HER2 over expression may confer
only partial resistance to endocrine therapies, and this effect
may be more evident with tamoxifen rather than the aromatase inhibitors.
Balducci L. The geriatric cancer patient: Equal benefit from
equal treatment. Cancer Control 2001;8(2 Suppl):1-25.
Full-Text
Du X, Goodwin JS. Patterns of use of chemotherapy for breast
cancer in older women: Findings from Medicare claims data. J
Clin Oncol 2001;19:1455-61. Abstract
Hurria A et al. Factors influencing treatment patterns of breast
cancer (BC) patients (Pts) age 75 and older. Proc
ASCO 2001; Abstract
1577.
Kimmick GG, Muss HB. Systemic therapy for older women with
breast cancer. Oncology (Huntingt) 2001;15(3):280-91.
Abstract
Lichtman SM, Villani G. Chemotherapy in the elderly: Pharmacologic
considerations. Cancer Control 2000;7(6):548-56. Full-Text
| Combined
Use of Aromatase Inhibitors and
Ovarian Ablation for Metastatic Breast Cancer |
|
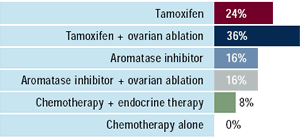
| Oncologists: Have you utilized
an aromatase inhibitor in combination with ovarian ablation?

Miami meeting attendees: What therapy would
you recommend for a 43-year-old premenopausal woman
with ER-positive, HER2-negative, asymptomatic bone metastases
who received no adjuvant therapy?
|
|
 |
 |
Clinical data have demonstrated an advantage for LHRH agonists
plus tamoxifen when compared to LHRH agonists alone in premenopausal
patients with metastatic disease. Unfortunately, there are no data
comparing the combination to tamoxifen alone. Among oncologists
surveyed, over one-third would treat a premenopausal woman with
an LHRH agonist/tamoxifen regimen, and one-quarter would utilize
tamoxifen alone. Interestingly, nearly 20% would combine an aromatase
inhibitor with ovarian ablation to treat a 43-year-old patient with
asymptomatic bone metastases — a maneuver that has been utilized
by 70% of oncologists in certain patients at some point in their
practice.
Celio L et al. Premenopausal breast cancer patients treated
with a gonadotropin-releasing hormone analog alone or in combination
with an aromatase inhibitor: A comparative endocrine study. Anticancer
Res 1999;19:2261-8. Abstract
Cheung KLet al. The combined use of goserelin and anastrozole
as second-line endocrine therapy in premenopausal women with advanced
breast cancer — a study of its clinical and endocrine effects.
Proc ASCO 2001; Abstract
1937.
Forward D et al. Combined use of goserelin (Zoladex) and anastrozole
(Arimidex) in premenopausal women with metastatic breast cancer
(MBC). Proc ASCO 2000; Abstract
582.
Ingle JN et al. Combination hormonal therapy involving aromatase
inhibitors in the management of women with breast cancer. Endocr
Relat Cancer 1999;6:265-9. Abstract
| Dosing
and Scheduling of Capecitabine |
|
| Oncologists: What dosing schedule
for capecitabine do you generally use for a 55-year-old
patient?

Oncologists: What dosing schedule for capecitabine
do you generally use for a 75-year-old patient?

|
|
While 2500 mg/m2/day is the approved dose for capecitabine, retrospective
studies have demonstrated no decrement in response associated with
reducing the dose to 2000 mg/m2/ day. While nearly half of respondents
would start a 55-year-old woman at full-dose capecitabine, three-quarters
would reduce this to 2000 mg/m2/day for a 75 - year-old woman. A
current adjuvant capecitabine trial in elderly women will utilize
this reduced dose. The primary dose-limiting toxicity for capecitabine
use is hand-foot syndrome.
Fujimoto-Ouchi K et al. Schedule dependency of antitumor activity
in combination therapy with capecitabine/5’-deoxy-5-fluorouridine
and docetaxel in breast cancer models. Clin Cancer Res
2001;7(4):1079-86. Abstract
Michaud LB et al. Improved therapeutic index with lower-dose
capecitabine in metastatic breast cancer (MBC) patients (Pts). Proc
ASCO 2000; Abstract
402.
O’Shaughnessy J. Clinical experience of capecitabine in
metastatic breast cancer. Eur J Cancer 2002;38 Suppl
2:10-4. Abstract
O’Shaughnessy J et al. A retrospective evaluation of the
impact of dose reduction in patients treated with Xeloda (capecitabine).
Proc ASCO 2000; Abstract
400.
| Dosing
and Scheduling of Capecitabine |
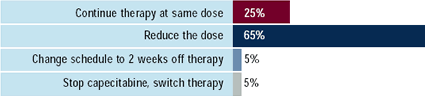
|
| Oncologists: A 55-year-old,
asymptomatic woman with lung metastases was started
on capecitabine at 2000 mg/m2/day given in two divided
doses for 2 weeks on, then one week off.
Scenario 1: After 3 cycles, there is no change
in the lesions and no side effects of therapy. What
would you generally do?

Scenario 2: After 3 cycles, there is an objective
response in the lung lesions, but the patient complains
of mild pain and redness in her hands and feet. What
would you generally do?
|
|
In a patient who is tolerating capecitabine but not responding,
25% of oncologists would dose-escalate. Although stable disease
in the asymptomatic patient with metastases is widely viewed as
a positive effect of therapy, about two-thirds of oncologists would
make some change in such a patient.
In the patient developing mild symptoms of hand-foot syndrome,
early intervention with dose reductions or changes in schedule are
usually recommended by research leaders, who also emphasize the
importance of educating patients to notify physicians about these
symptoms. Two-thirds of the oncologists report that less than half
of their patients on capecitabine develop side effects requiring
some type of intervention.
O’Shaughnessy J. Clinical experience of capecitabine in
metastatic breast cancer. Eur J Cancer 2002;38 Suppl
2:10-4. Abstract
| Tumor
Markers in Clinical Practice |
|
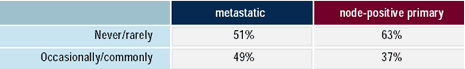
| Miami meeting attendees: Do
you use tumor markers for follow-up in the following
situations?
|
|
Over one-third of physicians surveyed utilize tumor markers to
detect recurrence after primary therapy in patients with node-positive
disease. Although there is one well-designed study, which demonstrated
that CA 27.29 could predict recurrence 5.3 months before other symptoms
or tests, the 2000 update to the American Society of Clinical Oncology
guidelines for the use of tumor markers does not recommend their
routine use in this setting, because the clinical benefit and options
for therapy are not significantly impacted. Another consideration
in determining whether or not to use tumor markers in this situation
is the psychological effect on the patient of rising tumor levels.
One-half of physicians reported the use of tumor markers in following
a patient with metastatic breast cancer. The ASCO guidelines do
not support the routine use of CEA to monitor response to treatment;
however, CEA may be useful in determining t reatment failure for
patients without readily measurable disease or those with elevated
CA 15-3 and/or CA 27.29.
Bast RC Jr et al. 2000 update of recommendations for the use
of tumor markers in breast and colorectal cancer: Clinical practice
guidelines of the American Society of Clinical Oncology.
J Clin Oncol 2001;19(6):1865-78. Abstract
Cheung KLet al. Tumour marker measurements in the diagnosis
and monitoring of breast cancer. Cancer Treat Rev 2000;26(2):91-102.
Abstract
Duffy MJ. Clinical uses of tumor markers: A critical review.
Crit Rev Clin Lab Sci 2001; 38 (3):225-62. Abstract
Nicolini A, Carpi A. Postoperative follow-up of breast cancer
patients: Overview and progress in the use of tumor markers.
Tumour Biol 2000;21(4):235-48. Abstract
Sakorafas GH et al. Follow-up after primary treatment for breast
cancer. Acta Oncol 2000;39(8):935-40. Abstract
|
