| You are here: Home: BCU 4|2002: Program Supplement: Adjuvant systemic therapy
|
Adjuvant
Endocrine Therapy in Postmenopausal Women:
Impact of the Early ATAC Trial Results
|
|
|
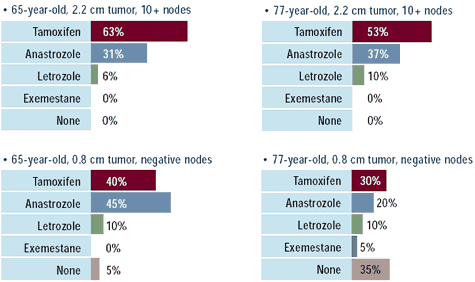
Oncologists: Which adjuvant endocrine therapy
would you recommend for the following patients with
ER-positive, HER2-negative breast cancer?
|
|
Just a few weeks after the presentation of the early ATAC trial
results, anastrozole was rapidly incorporated into the adjuvant
setting. In some clinical situations, almost half of oncologists
surveyed state that they are utilizing adjuvant anastrozole. While
some physicians may propose letrozole or exemestane, the vast majority
employing an adjuvant aromatase inhibitor prefer anastrozole.
Also note that while almost all of these women with ER-positive
disease receive some form of endocrine therapy, a significant minority
of oncologists would not prescribe endocrine therapy for elderly,
low-risk women.
Pharmacokinetics of anastrozole and tamoxifen alone and in
combination during adjuvant endocrine therapy for early breast cancer
in postmenopausal women: A sub-protocol of the “Arimidex®
and Tamoxifen Alone or in Combination” (ATAC) trial. Br
J Cancer 2001;85(3):317-324. Abstract
Baum M. The ATAC (Arimidex, Tamoxifen, Alone or in Combination)
adjuvant breast cancer trial in post-menopausal women. Breast
Cancer Res Treat 2001;69(3): Abstract
8.
Buzdar AU. Anastrozole (Arimidex) —- an aromatase inhibitor
for the adjuvant setting? Br J Cancer 2001;85(2 suppl):6-10.
Abstract
Goss PE. Preliminary data from ongoing adjuvant aromatase inhibitor
trials. Clin Cancer Res 2001;7(12 suppl):4397s-4401s.
Abstract
|
Adjuvant
Endocrine Therapy: Current and Future Use of Aromatase
Inhibitors
|
|
|
Miami meeting attendees: How is anastrozole
utilized as adjuvant therapy in postmenopausal patients
at the current time?

Miami meeting attendees: In three years, what
will be the most common adjuvant endocrine therapy of
postmenopausal women?

Miami meeting attendees: Do you believe that
the other aromatase inhibitors ( letrozole, exemestane)
can be used interchangeably with anastro zole as adjuvant
therapy?

|
|
Almost two-thirds of the Miami Breast Cancer Conference attendees
believe that in 2002, anastrozole will be utilized a great deal
as adjuvant endocrine therapy for postmenopausal breast cancer patients.
Faculty members Drs Richard Margolese and Patrick Borgen concur
with this belief. Of note, nearly 20% believe anastrozole will almost
completely replace tamoxifen in these patients this year.
This viewpoint was confirmed in physicians’ predictions for
clinical practice thre e years from now, with nearly three-quarters
stating that anastrozole will be the most commonly utilized adjuvant
endocrine therapy. Interestingly, there is a lack of support of
the other aromatase inhibitors as adjuvant therapy. This is likely
to continue until compelling, randomized clinical trial data become
available for these agents.
Nicholls H. Aromatase inhibitors continue their ATAC on tamoxifen.
Trends Mol Med 2002; 8 (4):S12-3. Abstract
|
Aromatase
Inhibitors in the Adjuvant Setting
|
|
|
Surgeons: If the ATAC data are widely accepted
and anastrozole generally replaces tamoxifen as adjuvant
endocrine therapy for postmenopausal women, which of
the following best describes how likely it is that surgeons
will prescribe anastrozole?

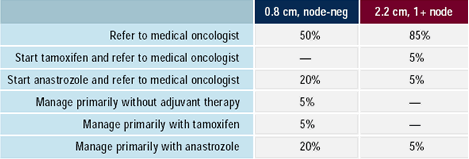
Surgeons: How would you manage the following
65-year-old woman with ER-positive invasive breast cancer?
|
|
 |
 |
With the increased use of chemotherapy in women with invasive breast
cancer, many surgeons routinely refer patients for evaluation by
a medical oncologist. However, it is also a common practice for
surgeons to initiate adjuvant endocrine therapy with tamoxifen.
The patterns of care survey demonstrates that this practice is also
likely to occur with adjuvant aromatase inhibitors in postmenopausal
patients. Surgeons are more likely to initiate adjuvant endocrine
therapy in lower-risk patients, who are less likely to receive adjuvant
chemotherapy. Almost one-third of Surgeons would manage an older
patient with a small, node-negative tumor without referral to a
medical oncologist.
Baum M. The ATAC (Arimidex, Tamoxifen, Alone or in Combination)
adjuvant breast cancer trial in post-menopausal women. Breast
Cancer Res Treat 2001;69(3): Abstract
8.
Buzdar AU. Anastrozole (Arimidex) — an aromatase inhibitor
for the adjuvant setting?
Br J Cancer 2001;85(2 suppl):6-10. Abstract.
|
Adjuvant
Endocrine Therapy in Premenopausal Women
|
|
|
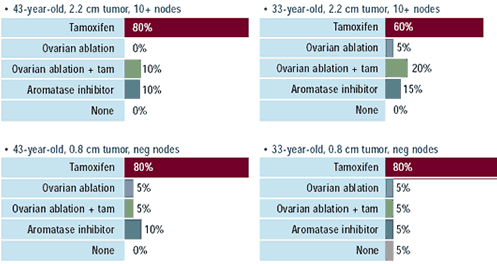
Oncologists: Which adjuvant endocrine therapy
would you recommend for the following patients with
ER-positive, HER2-negative breast cancer who continue
to menstruate after receiving chemotherapy?
|
|
 |
 |
Nearly all physicians would recommend some form of adjuvant endocrine
therapy for premenopausal women with ER-positive cancers, and tamoxifen
remains the common choice. Only a small fraction of physicians utilize
LHRH agonists or surgical oophorectomy despite findings from an
international meta-analysis demonstrating a significant survival
advantage for this intervention. A small fraction of oncologists
recommend aromatase inhibitors for premenopausal women; however,
these agents are only indicated for postmenopausal women. The encouraging
early results of the ATAC trial in postmenopausal patients has led
to the development of new randomized clinical studies evaluating
LHRH agonists plus aromatase inhibitors in premenopausal patients
with ER-positive tumors. Until results from these studies a re available,
most clinical researchers generally do not advocate the nonprotocol
use of this combined approach .
 |
 |
Ovarian ablation for early breast cancer. Early Breast Cancer
Trialists’Collaborative Group. Cochrane Database Syst
Rev. 2000:CD000485. Abstract
Davidson NE. Ovarian ablation as adjuvant therapy for breast
cancer. J Natl Cancer Inst Monogr 2001;30:67-71. Full
Text
|
Assessment
of ER and HER2 Status
|
|
|
Oncologists: How do you define ER-positivity?

Oncologists: Which of the following lab results
would you interpret as HER2-positive?

Oncologists: How often do you obtain FISH results
on your patients?

|
|
A widely quoted study by Harvey et al found that even those breast
cancer patients with 1-2% of cells with weak tumor ER staining benefit
from adjuvant hormonal therapy. All red has questioned the quality
control for the performance of this assay and assay interpretation
by clinicians managing breast cancer patients. While half of responding
oncologists treat breast cancers as ER-positive if they have any
staining on immunohistochemistry, another half utilize a laboratory-defined
cut-off for this definition.
A common algorithm for determining HER2 status, which is supported
by the NCCN, accepts an immunohistochemistry score of 3+ as positive,
but recommends FISH testing in tumors with an IHC score of 2+. This
approach is reflected in data from the oncologists’ survey.
Only 35% commonly obtain FISH testing in their patients.
Harvey JM et al. Estrogen receptor status by immunohistochemistry
is superior to the ligand-binding assay for predicting response
to adjuvant endocrine therapy in breast cancer. J Clin Oncol
1999;17:1474-81. Abstract
Perez EA et al. HER2 testing in patients with breast cancer:
Poor correlation between weak positivity by immunohistochemistry
and gene amplification by fluorescence in situ hybridization.
Mayo Clin Proc 2002;77(2):148-54. Abstract
|
Choice
of Adjuvant Chemotherapy
|
|
|
Miami meeting attendees: Do you think six cycles
of FAC is more effective
than four cycles of AC?

Oncologists: Which adjuvant chemotherapy regimen
would you use in the following 43-year-old women with
ER-negative, HER2-negative breast cancer?
|
|
There was considerable discussion at the 2000 NIH Consensus Conference
on the benefits of 4 cycles of AC chemotherapy compared to a longer
duration. Dr Gabriel Hortobagyi argued that indirect evidence supported
the use of a longer duration, but the Consensus statement reflected
that there was inadequate data to make this a standard recommendation.
Although a large proportion of physicians (including Drs Miller
and Mamounas) believe that 6 cycles of FAC is more effective than
4 cycles of AC, the addition of a taxane to the AC regimen was a
more common treatment choice. Clearly, in the higher-risk patient,
the vast majority of physicians believed AC chemotherapy to be inadequate
treatment.
National Institutes of Health Consensus Development Conference
statement: Adjuvant therapy for breast cancer, November 1-3, 2000.
J Natl Cancer Inst Monogr 2001;(30):5-15. Abstract
Aapro MS. Adjuvant therapy of primary breast cancer: A review
of key findings from the 7th international conference, St. Gallen,
February 2001. Oncologist 2001;6(4):376-85. Abstract
Hortobagyi GN. Progress in systemic chemotherapy of primary
breast cancer: An overview. J Natl Cancer Inst Monogr
2001;(30):72-9. Abstract
Shulman LN. What is the ideal duration of adjuvant therapy
for primary breast cancer: Are four cycles of cyclophosphamide and
doxorubicin enough? Curr Oncol Rep 2001;3(6):523-8. Abstract
|
Use
of Taxanes in the Adjuvant Setting
|
|
|
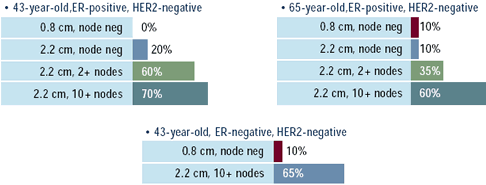
Oncologists: Would you use adjuvant taxanes
in the following patients?
(percent answering yes)
|
|
Although the 2000 NIH Consensus Conference on adjuvant therapy
of early breast cancer did not reach a consensus on the issue of
adjuvant taxanes, community practitioners use adjuvant taxanes frequently,
especially in women with high-risk tumors. The impact of age appears
to be greatest in the women with intermediate risk, with 60% of
oncologists treating a 43-year-old woman with a 2.2 cm tumor and
2+ nodes with taxanes, while only 35% would treat the 65-year-old
with similar risk. The impact of ER status appears negligible, despite
an unplanned re t rospective subset analysis in a large Interg roup
trial demonstrating less benefit of paclitaxel in women with ER-positive
than ER-negative tumors.
Mamounas EP, Sledge GW Jr. Combined anthracycline-taxane regimens
in the adjuvant setting. Semin Oncol 2001;28(4 Suppl
12):24-31. Abstract
Nabholtz JM, Riva A. The choice of adjuvant combination therapies
with taxanes: Rationale and issues addressed in ongoing studies.
Clin Breast Cancer 2001;2 Suppl 1:S7-S14. Abstract
Norton L. Theoretical concepts and the emerging role of taxanes
in adjuvant therapy. Oncologist 2001;6 Suppl 3:30-5.
Abstract
Piccart MJ et al. Taxanes in the adjuvant treatment of breast
cancer: Why not yet? J Natl Cancer Inst Monogr 2001;(30):88-95.
Abstract
Sparano JA. Taxanes for breast cancer: An evidence-based review
of randomized phase II and phase III trials. Clin Breast
Cancer 2000:32-40. Abstract
|
HER2
Status in Determining Adjuvant Therapy
|
|
|
Miami meeting attendees: Should HER2 status
be considered in deciding whether or not to use the
following agents in the adjuvant setting?
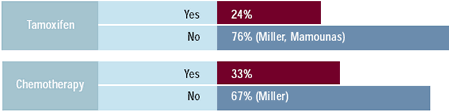
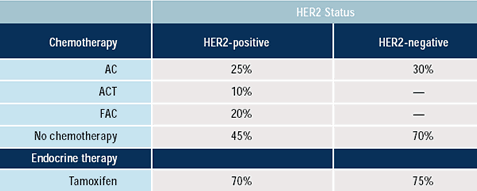
Oncologists: Which adjuvant therapy would you
give to a 43-year-old woman with a 0.8 cm, ER-positive,
node negative breast cancer based on HER2 status?
|
|
 |
 |
Most physicians state that they do not believe that decisions regarding
whether to use adjuvant chemotherapy or endocrine therapy should
be influenced by HER2 status of the patient’s tumor. However,
there is a significant tendency for oncologists to choose less aggressive
or no cytotoxic therapy in patients who have HER2-negative as opposed
to HER2-positive tumors. Anthracycline-based chemotherapy is routinely
utilized in women with both HER2-positive and HER2-negative cancers
as adjuvant therapy. Choice of endocrine therapy, particularly tamoxifen,
is not influenced by HER2 status, which is consistent with the lack
of conclusive evidence on this question in a variety of clinical
trials.
Piccart M et al. The predictive value of HER2 in breast cancer.
Oncology 2001;61 Suppl 2:73-82. Abstract
Ravdin PM. Is her2 of value in identifying patients who particularly
benefit from anthracyclines during adjuvant therapy? A qualified
yes. J Natl Cancer Inst Monogr 2001;30:80-4. Abstract
Sledge GW Jr. Is HER-2/neu a predictor of anthracycline utility?
No. J Natl Cancer Inst Monogr 2001;30:85-7. Abstract
|
Use
of Trastuzumab in the Adjuvant Setting
|
|
|
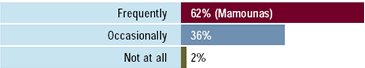
Miami meeting attendees: To what extent will
trastuzumab be used as adjuvant therapy for patients
with HER2-positive breast cancer 5 years from now?
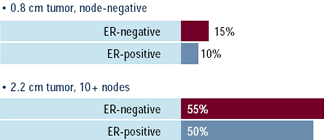
Oncologists: Would you currently use adjuvant
trastuzumab for the following 43-year-old patients with
HER2-positive disease? (percent answering yes)
|
|
The risks and benefits of adjuvant trastuzumab are currently under
investigation in several large, randomized clinical trials. Most
breast cancer research leaders believe that this intervention should
only be utilized in a research setting. However, when presented
with a young patient at high risk for recurrence because of multiple
positive nodes, about half of physicians would use adjuvant trastuzumab
if the tumor were HER2-positive. This practice seems to apply to
patients with both ER-positive and ER-negative tumors. A small but
significant number of physicians would also utilize trastuzumab
in lower-risk women.
Leyland-Jones B, Smith I. Role of Herceptin in primary breast
cancer: Views from North America and Europe. Oncology
2001;61 Suppl 2:83-91. Abstract
Nabholtz JM, Slamon D. New adjuvant strategies for breast cancer:
Meeting the challenge of integrating chemotherapy and trastuzumab
(Herceptin). Semin Oncol 2001;28(1 Suppl 3):1-12. Abstract
Slamon D, Pegram M. Rationale for trastuzumab (Herceptin) in
adjuvant breast cancer trials. Semin Oncol 2001;28(1
Suppl 3):13-9. Abstract
|
