| You are here: Home: BCU 4|2002: Program Supplement: Management of patients with HER-2 positive disease
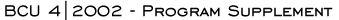
| Treatment
of Patients with ER-negative,
HER2-positive Metastatic Disease and Metastases
|
|
| Oncologists: How would you treat
the following patients with metastatic disease?
|
|
Virtually all breast cancer clinical investigators consider trastuzumab
a baseline to first-line therapy for women with HER2-positive metastatic
disease, as do the NCCN guidelines. However, a small but significant
fraction of oncologists do not follow this practice. Based on encouraging
reports from Vogel and others, a significant fraction of oncologists
uses trastuzumab monotherapy in the patient with metastases confined
to bone. Reflecting the pivotal randomized trial data from Slamon
et al demonstrating a survival advantage with the addition of trastuzumab
to paclitaxel, taxanes are most commonly the agents combined with
trastuzumab. However, physicians also utilize vinorelbine and capecitabine
in this situation.
Slamon DJ et al. Use of chemotherapy plus a monoclonal antibody
against HER2 for metastatic breast cancer that overexpresses HER2.
N Engl J Med 2001; 344 (11):783-92. Abstract
Vogel CL et al. Efficacy and safety of trastuzumab as a single
agent in first-line treatment of HER2-overexpressing breast cancer.
J Clin Oncol 2002;20(30):719-26. Abstract
| Trastuzumab
Use in the Patient with
HER2-positive, ER-positive Metastatic Disease |
|
| Oncologists: Which therapy would
you utilize at first relapse in the following women
with ER-positive, HER2-positive disease with bone metastases?
|
|
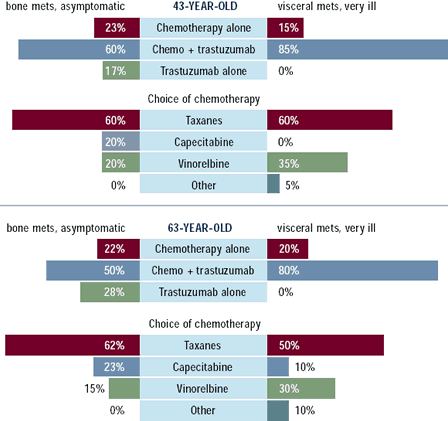
When treating patients with ER-positive, HER2-positive metastatic
disease with bone metastases, 40-80% of oncologists would utilize
hormonal therapy alone as first-line t reatment. A significant minority
would use hormonal therapy plus trastuzumab in these women. In the
43- and 63-year-old women, approximately 40% of oncologists would
combine chemotherapy with either trastuzumab or endocrine therapy.
Buzdar AU. Endocrine therapy in the treatment of metastatic
breast cancer. Semin Oncol 2001;28(3):291-304. Abstract
Hortobagyi GN. Overview of treatment results with trastuzumab
(Herceptin) in metastatic breast cancer. Semin Oncol 2001;28(6
Suppl 18):43-7. Abstract
Leyland-Jones B. Trastuzumab: Hopes and realities. Lancet
Oncol 2002;3(3):137-44. Abstract
McKeage K, Perry CM. Trastuzumab: A review of its use in the
treatment of metastatic breast cancer overexpressing HER2.
Drugs 2002;62(1):209-43. Abstract
Mouridsen H et al. Superior efficacy of letrozole versus tamoxifen
as first-line therapy for postmenopausal women with advanced breast
cancer: Results of a phase III study of the International Letrozole
Breast Cancer Group. J Clin Oncol 2001;19(10):2596-606.
Abstract
Nabholtz JM et al. Anastrozole is superior to tamoxifen as
first-line therapy for advanced breast cancer in postmenopausal
women: Results of a North American multicenter randomized trial.
Arimidex Study Group. J Clin Oncol 2000;18(22):3758-67.
Abstract
Robertson JF. Estrogen receptor downregulators: New antihormonal
therapy for advanced breast cancer. Clin Ther 2002;24
Suppl A:A17-30. Abstract
Winer EP, Burstein HJ. New combinations with Herceptin in metastatic
breast cancer. Oncology 2001;61 Suppl 2:50-7. Abstract
| Chemotherapy
and Trastuzumab in the Metastatic SettIng |
|
| Oncologists: Which therapy would
you utilize in the following patients with ER-negative,
HER2-positive asymptomatic bone metastases who have
not received prior adjuvant therapy?
|
|
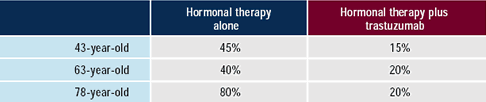
As patients age, the use of chemotherapy decreases, and the use
of single-agent trastuzumab in asymptomatic patients increases from
15% to 40% for the 43- and 78 - year-old women, respectively. Twenty-five
percent (25%) to 35% of physicians would utilize a single-agent
chemotherapy in these HER2-positive patients. Of the chemotherapy
selected, approximately half would utilize a taxane. A subset of
physicians would utilize more convenient and tolerable agents such
as capecitabine or vinorelbine especially in the 78-year-old woman.
Baselga J. Herceptin alone or in combination with chemotherapy
in the treatment of HER2-positive metastatic breast cancer: Pivotal
trials. Oncology 2001;61 Suppl 2:14-21. Abstract
Burstein HJ et al. Clinical activity of trastuzumab and vinorelbine
in women with HER2-overexpressing metastatic breast cancer.
J Clin Oncol 2001;19(10):2722-30. Abstract
Esteva FJ et al. Phase II study of weekly docetaxel and trastuzumab
for patients with HER-2-overexpressing metastatic breast cancer.
J Clin Oncol 2002;20(7):1800-8. Abstract
Miller KD et al. Gemcitabine, paclitaxel, and trastuzumab in
metastatic breast cancer. Oncology (Huntingt) 2001;15(2
Suppl 3):38-40. Abstract
Pegram MD. Docetaxel and Herceptin: Foundation for future strategies.
Oncologist 2001;6 Suppl 3:22-5. Abstract
Seidman AD et al. Weekly trastuzumab and paclitaxel therapy
for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype
and gene amplification. J Clin Oncol 2001;19(10):2587-95.
Abstract
Slamon DJ et al. Use of chemotherapy plus a monoclonal antibody
against HER2 for metastatic breast cancer that overexpresses HER2.
N Engl J Med 2001; 344 (11):783-92. Abstract
| Management
of the Patient with
HER2 - positive Breast Cancer |
|
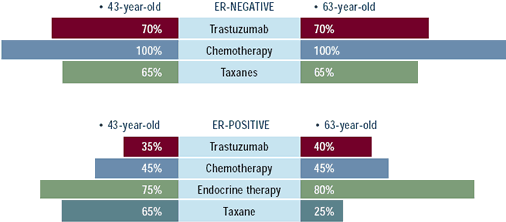
| Oncologists: How would you treat
the following women with HER2-positive disease, bone
metastases and multiple sites of moderate pain who have
not received adjuvant therapy?
|
|
 |
 |
Although trastuzumab is considered standard baseline therapy for
patients with HER2-positive metastatic disease by most breast cancer
researchers and the NCCN guidelines, about one-third of oncologists
do not routinely follow this practice. Management of patients with
ER-positive, HER2-positive disease is controversial. Current clinical
trials are evaluating the use of anastrozole plus trastuzumab in
postmenopausal patients, and there is a theoretical rationale to
combine these two non-cross-resistant and potentially complementary
antitumor approaches. Until the results of these trials are available,
many physicians will initiate endocrine treatment and utilize trastuzumab
at the time of progression, but about one-third of oncologists are
combining trastuzumab and endocrine therapy.
 |
 |
Knoop AS et al. Value of epidermal growth factor receptor, HER2,
p53, and steroid receptors in predicting the efficacy of tamoxifen
in high-risk postmenopausal breast cancer patients. J Clin
Oncol 2001;19(14):3376-84. Abstract
Lipton A et al. Elevated serum Her-2/neu level predicts decreased
response to hormone therapy in metastatic breast cancer. J
Clin Oncol 2002;20(6):1467-72. Abstract
Winer EP, Burstein HJ. New combinations with Herceptin in metastatic
breast cancer. Oncology 2001;61 Suppl 2:50-7. Abstract
Yamauchi H et al. When is a tumor marker ready for prime time?
A case study of c-erbB-2 as a predictive factor in breast cancer.
J Clin Oncol 2001;19(8):2334-56. Abstract
| Trastuzumab
in the Metastatic Setting |
|
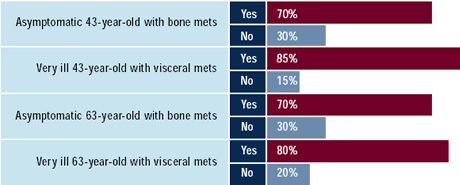
| Oncologists: Would you use trastuzumab
in the following women with ER-negative, HER2-positive
metastatic disease, who have received adjuvant ACT?
|
|
The use of trastuzumab in the metastatic setting appears to be
slightly greater in symptomatic than asymptomatic disease and greater
in women who have re c e i v e d adjuvant ACT than those who received
no adjuvant therapy. This effect is independent of the patient’s
age.
Bell R. Ongoing trials with trastuzumab in metastatic breast
cancer. Ann Oncol 2001;12 Suppl 1:S69- 73. Abstract
Cobleigh M et al. Multinational study of the efficacy and safety
of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing
metastatic breast cancer that has progressed after chemotherapy
for metastatic disease. J Clin Oncol 1999;17(9):2639-48.
Abstract
Cook-Bruns N. Retrospective analysis of the safety of Herceptin
immunotherapy in metastatic breast cancer. Oncology 2001;61
Suppl 2:58-66. Abstract
Slamon DJ et al. Use of chemotherapy plus a monoclonal antibody
against HER2 for metastatic breast cancer that overexpresses HER2.
N Engl J Med 2001; 3 4 4 (11):783-92. Abstract
Vogel CL et al. Efficacy and safety of trastuzumab as a single
agent in first-line treatment of HER2-overexpressing metastatic
breast cancer. J Clin Oncol 2002;20(3):719-26. Abstract
Winer EP, Burstein HJ. New combinations with Herceptin in metastatic
breast cancer. Oncology 2001;61 Suppl 2:50-7. Abstract
| Duration
of Chemotherapy and
Trastuzumab in Metastatic Disease |
|
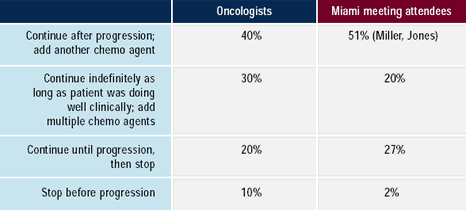
| A 57-year-old patient with HER2-positive
breast cancer is treated with trastuzumab /paclitaxel
on first relapse for bone metastases. After 4 months,
she has had a good response and is doing well. Which
of the following best describes how long you would normally
continue trastuzumab?
|
|
Although no definitive data exist on the optimal duration of trastuzumab
therapy for metastatic disease, most oncologists and attendees polled
at the 2002 Miami Breast Cancer Conference continue trastuzumab
after disease progression and add another chemotherapeutic agent.
Several current clinical trials are evaluating the optimal duration
for trastuzumab, specifically whether to continue treatment beyond
progression.
Bell R. Duration of therapy in metastatic breast cancer: Management
using Herceptin. Anticancer Drugs 2001;12(7):561-8. Abstract
Hortobagyi GN. Optimal duration of therapy with trastuzumab.
Semin Oncol 2001;28(5 Suppl 16): 33-40. Abstract
Leyland-Jones B. Trastuzumab: Hopes and realities. Lancet
Oncol 2002;3(3):137-44. Abstract
| Trastuzumab
and Cardiotoxicity |
|
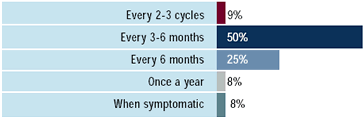
| Oncologists: How often is cardiotoxicity
a major concern when considering the use of trastuzumab
for metastatic disease?

Oncologists: What is your routine
interval of cardiac monitoring in patients who receive
trastuzumab?
|
|
In the trastuzumab pivotal trials, cardiac dysfunction (CD) was
observed, particularly in patients treated with anthracyclines.
The pathophysiologic basis for trastuzumab associated CD remains
unclear. Formal safety analyses have been introduced into large
randomized trials conducted by the NSABP, Intergroup and other cooperative
groups.
While many believe that the risk of trastuzumab-associated CD
is justifiable in the metastatic setting, three-quarters of oncologists
in the survey report occasional-to-frequent concerns about cardiotoxicity
when utilizing trastuzumab in patients with metastatic disease.
Sixty percent (60%) of oncologists report routine monitoring of
cardiac functioning. The MUGA scan is used by all oncologists, although
this procedure may not be able to identify CD before significant
cardiac damage has occurred. Other methods to monitor cardiac functioning
are being evaluated in clinical trials.
Seidman Aet al. Cardiac dysfunction in the trastuzumab clinical
trials experience. J Clin Oncol 2002;20(5):1215-21. Abstract
Chien KR. Myocyte survival pathways and cardiomyopathy: Implications
for trastuzumab cardiotoxicity. Semin Oncol 2000;27(6
Suppl 11):9-14. Abstract
Gerber B et al. Effectiveness of Trastuzumab (Herceptin) in
a patient with locally recurrent breast cancer after cardiac failure
caused by severe cytotoxic pretreatment. Oncology 2001;61(4):271-4.
Abstract
Sparano JA. Cardiac toxicity of trastuzumab (Herceptin): Implications
for the design of adjuvant trials. Semin Oncol 2001;28(1
Suppl 3):20-7. Abstract
|
