| |
Edith A Perez, MD |
EDITED COMMENTS |
 Editor’s Note: Editor’s Note:
Shortly after the interview with Dr Perez, the NCI issued a press release regarding the combined analysis of NCCTG-N9831 and NSABP-B-31, which evaluated AC followed by paclitaxel with or without trastuzumab in the adjuvant setting (see page 25, 5.1).
Overview of the adjuvant trials of trastuzumab
There are four very large trastuzumab adjuvant trials, which are complementary and reflect worldwide collaboration. The NSABP originally had overall survival as the primary endpoint. Then, based on new data, they changed the schedule of paclitaxel to be similar to what we did in the Intergroup study in 9831, in that they allowed weekly paclitaxel. They also modified their primary endpoint to be disease-free survival, which is consistent with our study. These two studies were built on what we had learned from CALGB-9344 and what we had learned in terms of tolerability from our own metastatic studies and even ECOG-E1199.
N-9831 includes patients with node-positive or high-risk, node-negative, HER2- positive breast cancer. The patients are randomly assigned to either: (1) chemotherapy alone, which consists of AC once every three weeks for four cycles, followed by weekly paclitaxel for 12 doses; (2) the same chemotherapy, followed by a year of weekly trastuzumab; or (3) the same chemotherapy with the introduction of trastuzumab when the patients start receiving weekly paclitaxel (ie, 12 doses of trastuzumab in combination with weekly paclitaxel and 40 additional weeks of trastuzumab).
The BCIRG-006 and HERA trials drew from other experiences. BCIRG used AC followed by docetaxel once every three weeks as the standard chemotherapy arm. Then they added sequential trastuzumab. In the third arm, they looked at a nonanthracycline-containing regimen, which uses docetaxel/carboplatin once every three weeks with trastuzumab. HERA was completely different in that it only looked at the sequential introduction of trastuzumab after chemotherapy.
The patients completed the chemotherapy and were then randomized to no trastuzumab, trastuzumab for one year or trastuzumab for two years. In HERA, trastuzumab is administered once every three weeks. They have a detailed description of which chemotherapies are potentially to be included, but the list is very long. Essentially, it’s almost anything that physicians feel comfortable recommending to their patients.
Initial reporting of the adjuvant trials of trastuzumab
We have been working with the NSABP, the National Cancer Institute and the FDA for more than a year in order to obtain approval for a formal joint analysis of the NCCTG and NSABP trials of adjuvant trastuzumab for disease-free survival and overall survival. We recently obtained formal FDA approval. The next step will be to look at the first interim evaluation of the data.
There are enough events between the two trials to perform that analysis, but the question remains whether the magnitude of the difference between trastuzumab versus no trastuzumab is large enough to meet the statistical boundaries outlined for the release of the data. Although N9831 will complete accrual in a couple of months, we still need some time for all of the patients to receive trastuzumab, so we need to be very careful. We don’t want to be too premature in releasing data. At the same time, if the differences are large enough to cross statistical boundaries, then we would need to go to our respective Data Monitoring Committees and have discussions related to timing of release of this information.
The data could be available soon if the differences are huge. This is very exciting because there has been a lot of work and time invested in the correct conduct of these clinical trials with appropriate monitoring, and patients have been very compliant. The BCIRG study was closed to patient accrual almost a year ago and the HERA study completed accrual a few months ago. HERA enrolled more than 4,700 patients, and I believe the timing of data release may occur at approximately the same time for all of the trials because the combined analysis of N9831 with the NSABP study includes more than 5,000 patients.
Cardiac safety of adjuvant trastuzumab
When we designed N9831, it was a coordinated effort between many groups because we wanted to have consistent cardiac testing and definitions of what we considered to be clinically acceptable. More than a four percent difference in clinical cardiac events between the trastuzumab-containing and the nontrastuzumab- containing arms would have been considered unacceptable. Although our clinical trial demonstrated that clinical cardiac events are observed in patients receiving adjuvant trastuzumab, I’m pleased to say that the difference is less than four percent compared to the control arm (Perez 2005b). The numbers are actually a bit lower than the numbers in NSABP-B-31 (Geyer 2003) but statistically quite similar.
At this point, we have not seen any difference in cardiac events between the two trastuzumab-containing arms. Not every patient has a reversal of their cardiac events, but most patients definitely improve not only in terms the clinical symptomatology but also measurable left ventricular ejection fraction.
5.1 National Cancer Institute News Release, April 25, 2005 (excerpt)
http://www.nci.nih.gov/newscenter/pressreleases/HerceptinCombination2005
Trastuzumab Combined with Chemotherapy Improves Disease-Free Survival for Patients with Early-Stage Breast Cancer
“Results from two large randomized clinical trials for patients with HER-2 positive invasive breast cancer show that those patients with early-stage breast cancer who received Herceptin® (trastuzumab) in combination with chemotherapy had a significant decrease in risk for breast cancer recurrence compared with patients who received the same chemotherapy without trastuzumab...
“The Data Monitoring Committees overseeing the combined analysis of these trials (known as NSABP-B-31 and NCCTG-N9831) recommended that the results of a recent combined interim analysis be made public because the studies had met their primary endpoints of increasing disease-free survival...
“The improvement in overall survival also was statistically significant for women receiving a combination of chemotherapy and trastuzumab...
“Patients in the clinical trials who received trastuzumab in combination with standard combination chemotherapy had a 52 percent decrease in disease recurrence compared to patients treated with chemotherapy alone. This difference is highly statistically significant. ‘This is a major advance for many thousands of women with breast cancer,’ said NCI Director Andrew C von Eschenbach, MD. ‘These results are one more example that we are at a major turning point in the use of targeted therapies to eliminate suffering and death from cancer,’ he added.
“‘These findings confirm that we now have a very potent weapon against the recurrence of cancer cells that overexpress HER-2,’ said Edith A Perez, MD, who chaired the NCCTG trial and is a medical oncologist at the Mayo Clinic in Jacksonville, FL.
“Edward Romond, MD, study chair for the NSABP and professor of oncology at the University of Kentucky, in Lexington, KY, noted, ‘For women with this type of aggressive breast cancer, the addition of trastuzumab to chemotherapy appears to virtually reverse prognosis from unfavorable to good.’
“Information from over 3,300 patients enrolled in these studies was used for analysis. Patients with operable breast cancer whose tumors over-expressed HER-2 were enrolled in these studies between February 2000 and April 2005. Patients were randomized to receive chemotherapy with doxorubicin and cyclophosphamide followed by paclitaxel, or doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab.
“Chemotherapy of the type given in these studies has a risk of congestive heart failure (weakening of the heart muscle) of less than 1 percent. In these studies, the likelihood of congestive heart failure in women receiving the combination of chemotherapy and trastuzumab was increased by 3 Percent to 4 percent.” |
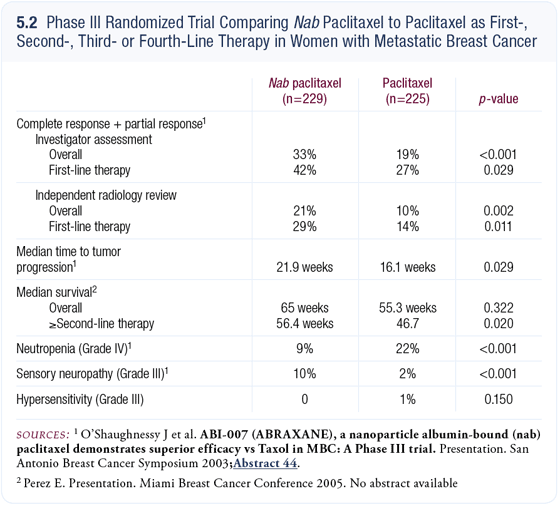
Phase III randomized trial comparing nanoparticle albumin-bound (nab) paclitaxel (Abraxane) to paclitaxel
Efficacy
Investigators enrolled patients who were eligible to receive first-, second-, thirdor even fourth-line chemotherapy for metastatic breast cancer. The data are mature, were presented at the 2003 San Antonio Breast Cancer Symposium and will soon be published. This trial demonstrated improvements in the response rate and time to progression for patients treated with nab paclitaxel compared to patients treated with paclitaxel, when both drugs were administered once every three weeks (O’Shaughnessy 2003; [5.2]).
We recently obtained the survival data from this study, which we presented at the 2005 Miami Breast Cancer Conference. In the overall group of patients, a 10-week improvement in median survival was found for the patients assigned to nab paclitaxel compared to patients treated with paclitaxel, but that number did not reach statistical significance.
However, when subset analyses were performed, patients treated with nab paclitaxel as second-, third- or fourth-line therapy still had a 10-week improvement in median survival compared to patients treated with paclitaxel, and the number reached statistical significance (Perez 2005a; [5.2]).
Toxicities
Despite premedications, some allergic reactions were seen in the patients treated with paclitaxel. We have seen this not only with paclitaxel but also with docetaxel. In spite of almost a doubling of the paclitaxel dose with nab paclitaxel (260 mg/m2 versus 175 mg/m2 both administered once every three weeks), a significantly lower incidence of myelosuppression was observed with nab paclitaxel than paclitaxel (O’Shaughnessy 2003; [5.2]).
More cases of neuropathy were seen in the patients treated with nab paclitaxel than in patients treated with paclitaxel; however, the numbers were small in both arms of the trial. The relative rates of Grade III sensory neuropathy were 10 percent for patients treated with nab paclitaxel and two percent for patients treated with paclitaxel (O’Shaughnessy 2003; [5.2]).
Because we’re administering more paclitaxel with the albumin-bound formulation, it’s not completely unexpected that we would see more neuropathy. The investigators evaluated the evolution of the neuropathy in the small group of patients. With treatment interruption, the Grade III neuropathy associated with nab paclitaxel resolved to Grade I or II after a median of 22 days (O’Shaughnessy 2003).
Select publications
 |
Dr Perez is a Professor of Medicine at the Mayo Medical School, Director of the Cancer Clinical Study Unit and Director of the Breast Cancer Program in the Division of Hematology and Oncology at the Mayo Clinic in Jacksonville, Florida. |
|
|

