You are here: Home: BCU 4 | 2005: Power Point Journal Club
| |
|
|
| |

This PowerPoint Journal reviews recently published clinical research articles and presentations. In this issue, we review a study by Aman Buzdar, MD and Cynthia Macahilig demonstrating the influence of clinical trial results from the ATAC trial over 28 months on the use of tamoxifen and anastrozole in the treatment of postmenopausal women with hormone receptor-positive early breast cancer; a report by Kathy Miller, MD et al on a Phase III trial comparing capecitabine with or without bevacizumab in patients with previously treated metastatic disease; and papers by Mehrdad Nadji, MD and colleagues evaluating IHC assays for estrogen and progesterone receptors in 5,993 cases of invasive mammary carcinomas.
These PowerPoint Journal Club slides are provided in different formats in this monograph and on the enclosed enhanced CD. The slide presentation on the CD was designed for optimal viewing on a large screen in a dark room (below, right) and represents top-line data and information from the figures in this book. The PowerPoint file and PDF file of this monograph can be accessed at www.BreastCancerUpdate.com.

| 6.1 |
|
| |
|
 
SLIDE 6.1 Results from large clinical trials should have an impact
on clinical practice. Relatively few studies, though, have assessed
the influence of clinical trial results on the practice of oncology.
|
| |
|
|
| 6.2 |
|
| |
|

SLIDE 6.2 In this paper, Buzdar and Macahilig attempt to determine
the impact of the presentation and publication of the results
from the ATAC trial on medical oncologists’ prescribing patterns
for adjuvant hormonal therapy in the United States.
|
| |
|
|
| 6.3 |
|
| |
|
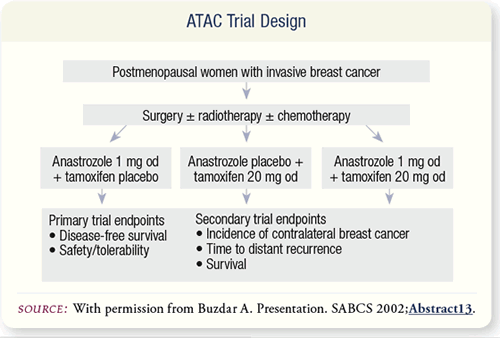
SLIDE 6.3 ATAC compared anastrozole, tamoxifen, or the combination
as adjuvant therapy in postmenopausal women (n=9,366)
with operable breast cancer. The primary endpoints for the trial
were disease-free survival and safety/tolerability.
|
| |
|
|
| 6.4 |
|
| |
|
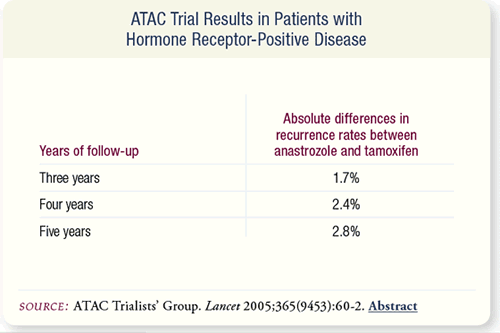
SLIDE 6.4 After a median follow-up of 68 months, there were significant
improvements in disease-free survival and time to recurrence
with anastrozole compared to tamoxifen. With hormonereceptor
positive disease, the absolute difference in recurrence
rates increased with each year of follow-up.
|
| |
|
|
| 6.5 |
|
| |
|
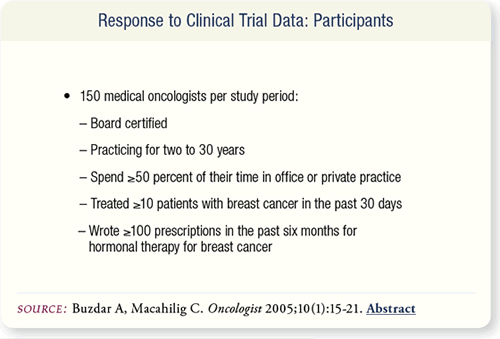
SLIDE 6.5 For each study period, 150 US oncologists were recruited.
Participants were board certified, spent at least 50 percent of
their time in office or private practice, treated at least 10 breast
cancer patients in the past 30 days and wrote at least 100 prescriptions
for hormone therapy in the past six months.
|
| |
|
|
| 6.6 |
|
| |
|
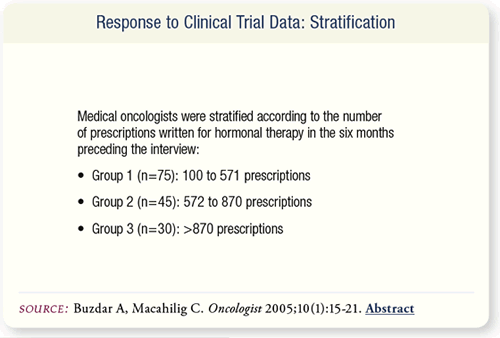
SLIDE 6.6 The medical oncologists were stratified by the number
of prescriptions they wrote for hormonal therapy for breast cancer
in the previous six months. Group 1 wrote 100 to 571 prescriptions.
Group 2 wrote 572 to 870 prescriptions, and Group 3 wrote
more than 870 prescriptions.
|
| |
|
|
| 6.7 |
|
| |
|
SLIDE 6.7 The data were collected via structured, computerassisted
telephone interviews that lasted 45 to 60 minutes. Eight
different study periods were used: July 2001, March 2002, July
2002, November 2002, February 2003, June 2003, August 2003 and
November 2003.
|
| |
|
|
| 6.8 |
|
| |
|
SLIDE 6.8 Predefined questions were utilized in the telephone
interviews. The medical oncologists were asked about their initial
hormonal therapy choices for the last five postmenopausal
patients with ER-positive, early breast cancer.
|
| |
|
|
| 6.9 |
|
| |
|
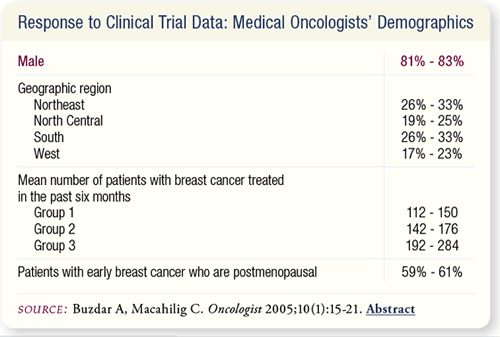
SLIDE 6.9 The majority of participants were males who were consistently
distributed across the four US geographic regions. Each medical
oncologist had treated a mean of 112 to 284 patients with breast
cancer in the last six months. They all indicated that about 60 percent
of their patients with early breast cancer were postmenopausal.
|
| |
|
|
| 6.10 |
|
| |
|
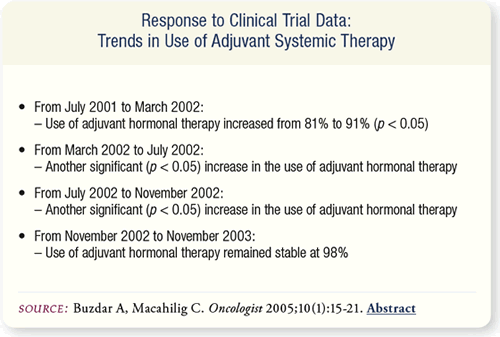
SLIDE 6.10 The reported use of adjuvant hormonal therapy for
postmenopausal women with ER-positive early breast cancer
increased from 81 percent in July 2001 to 98 percent in November
2003. During the same time, the use of adjuvant chemotherapy
remained relatively stable, ranging from 36 to 50 percent.
|
| |
|
|
| 6.11 |
|
| |
|
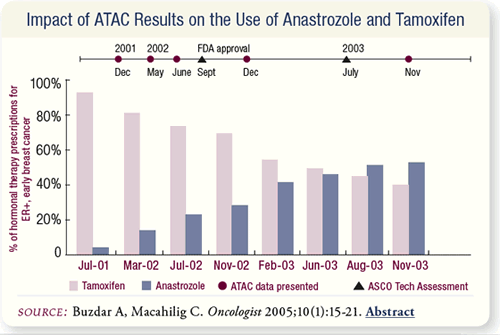
SLIDE 6.11 After the initial ATAC trial results were presented in
December 2001, the reported use of anastrozole increased from
two percent (July 2001) to 14 percent (March 2002) of adjuvant
hormonal therapies. By November 2003, anastrozole accounted
for 53 percent of the hormonal therapy choices.
|
| |
|
|
| 6.12 |
|
| |
|

SLIDE 6.12 The reports of the positive ATAC trial results appear to
have significantly influenced adjuvant hormonal therapy selection
as reported by medical oncologists in the United States.
Future trials should evaluate the influence of clinical trial results
on prescribing patterns.
|
| |
|
|
Select Publications
| 7.1 |
|
| |
|

SLIDE 7.1 In a Phase II trial of bevacizumab in patients previously
treated for metastatic breast cancer (MBC), the rates for objective
response and stable disease at 22 weeks were 9.3 and 17 percent,
respectively. Those benefits were the basis for a Phase III randomized
trial comparing capecitabine with or without bevacizumab.
|
| |
|
|
| 7.2 |
|
| |
|
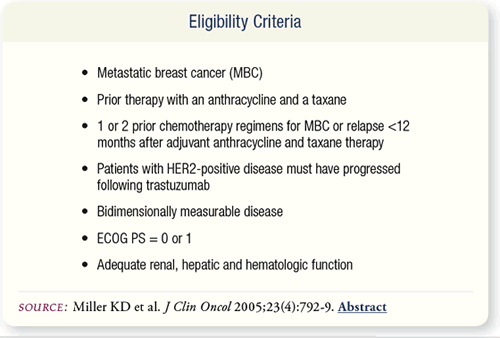
SLIDE 7.2 The eligibility criteria included MBC, prior anthracycline
and taxane, one or two prior chemotherapy regimens for MBC or
relapse <12 months after adjuvant anthracycline and taxane, progression
after trastuzumab for HER2-positive disease, ECOG PS = 0
or 1 and adequate renal, hepatic and hematologic function.
|
| |
|
|
| 7.3 |
|
| |
|
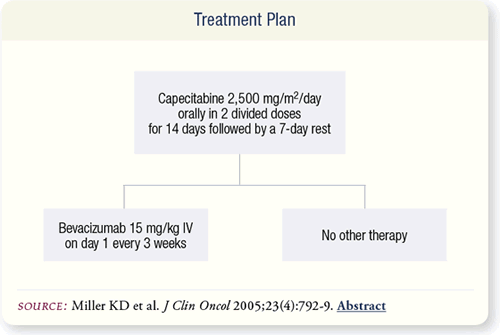
SLIDE 7.3 All patients received capecitabine 2,500 mg/m2/day
orally in two divided doses for 14 days followed by a seven-day
rest. Patients randomly assigned to the combination arm received
bevacizumab 15 mg/kg intravenously on day one of each threeweek
cycle. Therapy continued for a maximum of 35 cycles.
|
| |
|
|
| 7.4 |
|
| |
|
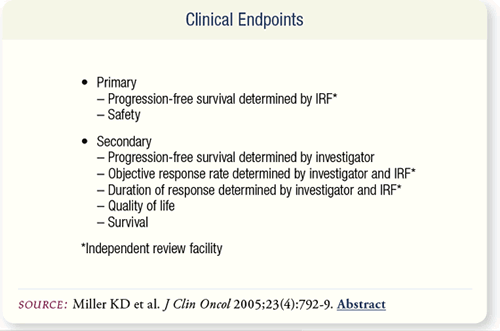
SLIDE 7.4 The primary endpoints of the trial were progressionfree
survival (PFS) and safety. Secondary endpoints included:
PFS, objective response rate, duration of response, quality of life
and survival.
|
| |
|
|
| 7.5 |
|
| |
|
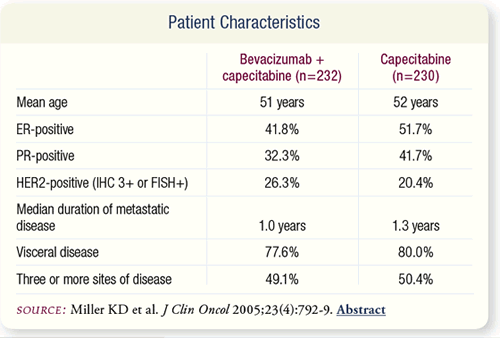
SLIDE 7.5 A total of 462 patients were enrolled on the trial. Two
hundred thirty patients were randomly assigned to capecitabine
alone and 232 to capecitabine plus bevacizumab. The patients’
baseline demographic and tumor characteristics were balanced
between the groups.
|
| |
|
|
| 7.6 |
|
| |
|
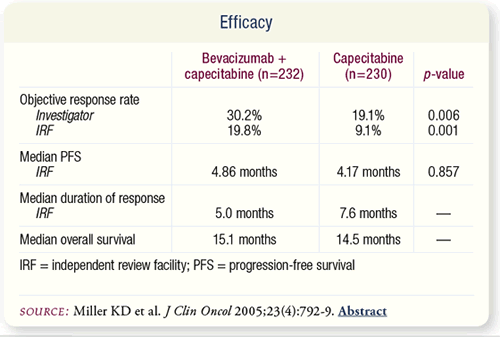
SLIDE 7.6 The addition of bevacizumab to capecitabine resulted
in significantly improved objective response rate. However, PFS,
response duration, overall survival or time to deterioration of
quality of life were not altered. The IRF and investigators disagreed
on disease progression in 105 patients.
|
| |
|
|
| 7.7 |
|
| |
|
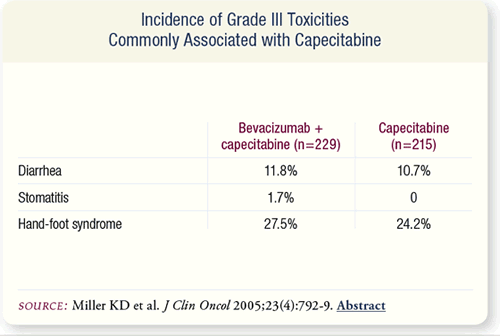
SLIDE 7.7 Bevacizumab did not alter the frequency or severity of
the Grade III toxicities associated with capecitabine.
|
| |
|
|
| 7.8 |
|
| |
|
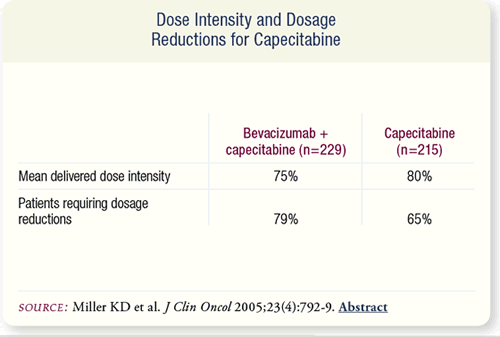
SLIDE 7.8 The mean delivered dose intensity for capecitabine
was similar for the patients in both randomization arms. Dosage
reductions for capecitabine were required for 65 percent of those
receiving capecitabine alone versus 79 percent of those receiving
the combination.
|
| |
|
|
| 7.9 |
|
| |
|
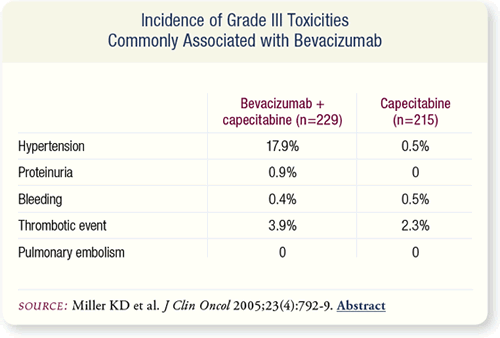
SLIDE 7.9 Grade III hypertension and proteinuria occurred more
frequently in patients receiving bevacizumab. Grade III bleeding
was rare and not different between groups, but patients receiving
capecitabine alone had fewer episodes of Grade I or II epistaxis.
|
| |
|
|
| 7.10 |
|
| |
|
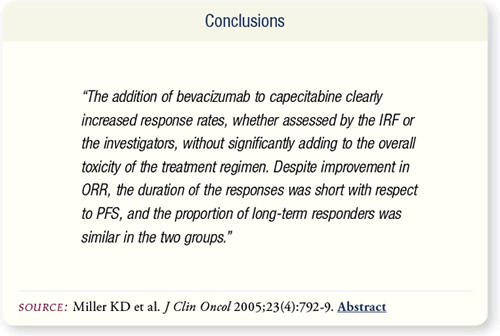
SLIDE 7.10 In women with previously treated MBC, the addition
of bevacizumab to capecitabine increases the response rate but
not PFS or response duration. Patients with less advanced disease
may obtain additional benefits from bevacizumab.
|
| |
|
|
| 7.11 |
|
| |
|
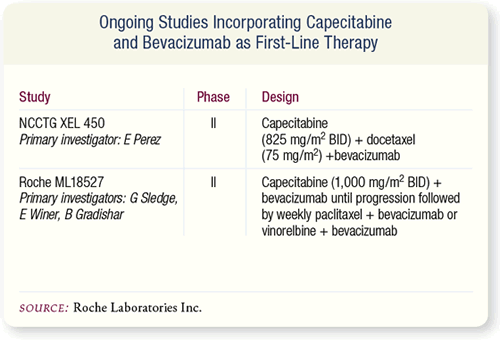
SLIDE 7.11 Currently, two Phase II trials are incorporating
capecitabine and bevacizumab into first-line chemotherapeutic
regimens for metastatic disease.
|
| |
|
|
Select publications
| 8.1 |
|
| |
|

SLIDE 8.1 In breast cancer, a positive estrogen receptor (ER) status has
correlated with disease-free survival and improved response with
hormone therapy. Immunohistochemical (IHC) analysis of ER status
may be more discriminatory for predicting overall and disease-free
survival in routine assessments of hormone receptor status.
|
| |
|
|
| 8.2 |
|
| |
|
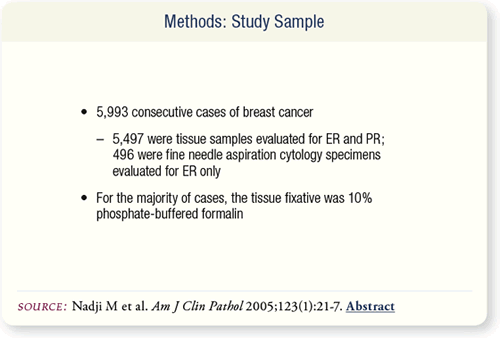
SLIDE 8.2 Initial observations from the daily IHC analysis of
steroid receptors in the laboratory led Nadji et al to re-evaluate
semiquantitation of the steroid receptor reaction. They conducted
a study of the immunohistochemical staining for ER and PR in
5,993 consecutive breast cancer cases during a six-year period.
|
| |
|
|
| 8.3 |
|
| |
|
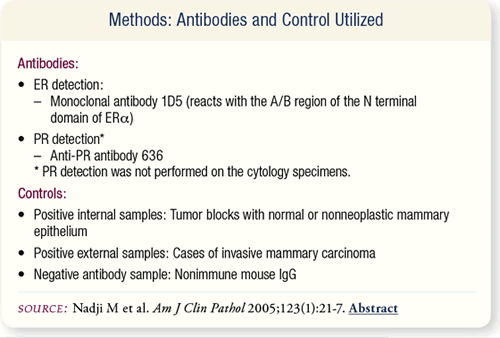
SLIDE 8.3 Mouse IgG monoclonal antibody (1D5) that reacts with
the A/B region of the N terminal domain of ERα was used to
detect ER. Monoclonal anti-PR antibody 636 was used to detect
PR. Positive and negative controls were also used.
|
| |
|
|
| 8.4 |
|
| |
|
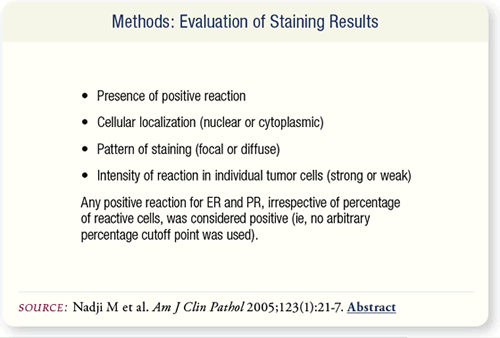
SLIDE 8.4 The stained slides were evaluated for the presence of
positive reaction, cellular localization, staining pattern (focal
or diffuse) and intensity of reaction in individual tumor cells
(strong or weak). Any positive reaction for ER and PR, irrespective
of percentage of reactive cells, was considered positive.
|
| |
|
|
| 8.5 |
|
| |
|
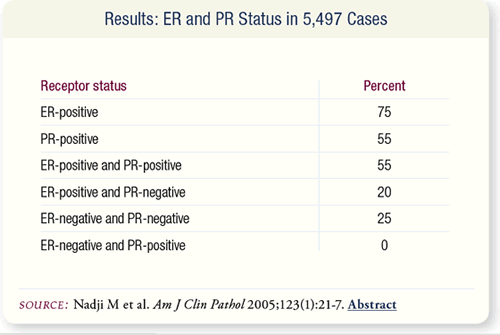
SLIDE 8.5 Of the tissue specimens, 75 percent were ER-positive, 55
percent were PR-positive, 55 percent were ER-positive/PR-positive,
20 percent were ER-positive/PR-negative and 25 percent were ERnegative/
PR-negative. All of the PR-positive specimens were also
ER-positive; no specimens were ER-negative and PR-positive.
|
| |
|
|
| 8.6 |
|
| |
|
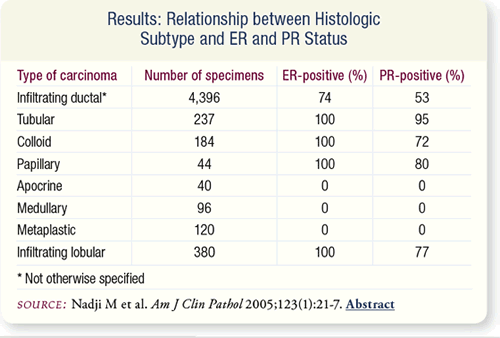
SLIDE 8.6 Among unspecified infiltrating ductal carcinoma specimens,
74 percent were ER-positive and 53 percent were PR-positive.
All pure tubular, colloid, papillary and infiltrating lobular
carcinomas and none of the apocrine, medullary or metaplastic
carcinomas were ER-positive. PR positivity was less predictable.
|
| |
|
|
| 8.7 |
|
| |
|
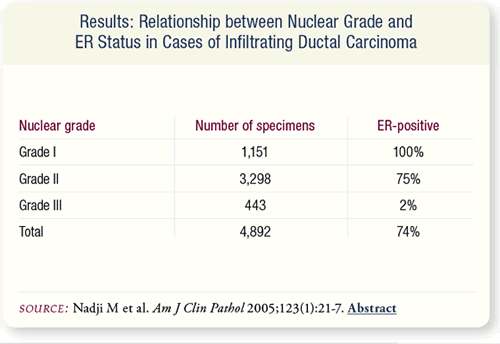
SLIDE 8.7 Among 4,892 cases of infiltrating ductal carcinoma (no
special type), all nuclear Grade I tumors were ER-positive. In contrast,
75 percent of the nuclear Grade II tumors and only two percent
of the nuclear Grade III tumors were ER-positive.
|
| |
|
|
| 8.8 |
|
| |
|
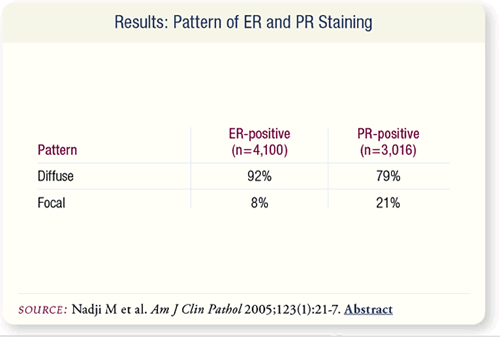
SLIDE 8.8 In most cases, positive staining for ER was diffuse. The
majority of the focal pattern observed in eight percent of ER-positive
cases was due to inadequate fixation or focal tumor necrosis.
Inadequate fixation did not account for the focal pattern of PR
staining, which was observed in 21 percent of PR-positive cases.
|
| |
|
|
| 8.9 |
|
| |
|

SLIDE 8.9 ER positivity and negativity are predictable in certain
histologic types and nuclear grades of breast cancer. With the ID5
monoclonal antibody and antigen retrieval, IHC staining for breast
cancer is an all-or-none occurrence, which is clinically relevant in
predicting survival. Quantitation of results is unnecessary.
|
| |
|
|
Select publications
|
|
| |
|
|
| |
|
|
| |
|
|
|
|

|
|
|

