William J Gradishar, MD |
EDITED COMMENTS |
 Phase II trial of capecitabine/paclitaxel as first-line therapy for metastatic disease Phase II trial of capecitabine/paclitaxel as first-line therapy for metastatic disease
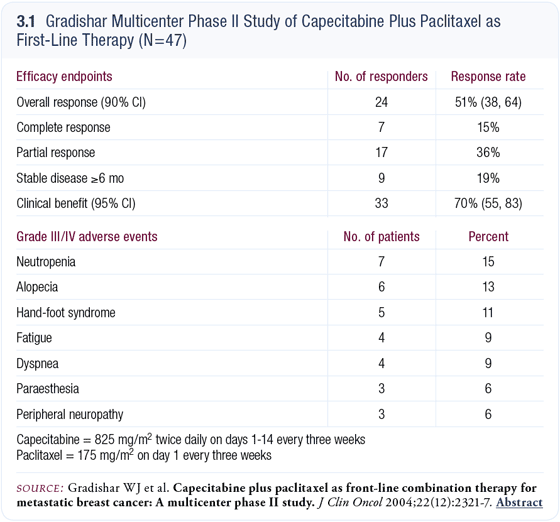
The rationale behind our study (Gradishar 2004) was to determine whether we could see a similar benefit to that observed in Joyce O’Shaughnessy’s docetaxel/capecitabine randomized trial (O’Shaughnessy 2002).
There were differences in the two trials. Our study was largely in the first line, whereas O’Shaughnessy’s trial had a mix of patients receiving first-, second- and third-line therapy.
The other distinction was the dose of the capecitabine. We started at 825 mg/m2 twice a day for 14 days out of 21 days as opposed to the FDA-approved dose (1,250 mg/m2) utilized in the other trial. We found the lower dose was better tolerated, which reflects the experience of most physicians using capecitabine as a single agent or in combination.
Dose reduction is usually necessary when starting at the FDA-approved dose. In practice, most physicians utilize one g/m2. So when combining with paclitaxel, the decision was made proactively that we would use a lower starting dose.
There was a very good response rate of approximately 50 percent (3.1), which is similar to O’Shaughnessy’s results in patients treated first line. If one is making the decision to combine capecitabine with a taxane, you could choose either docetaxel or paclitaxel and expect a robust response rate. It’s a reasonable combination if one is wedded to the idea of using a combination in a particular patient.
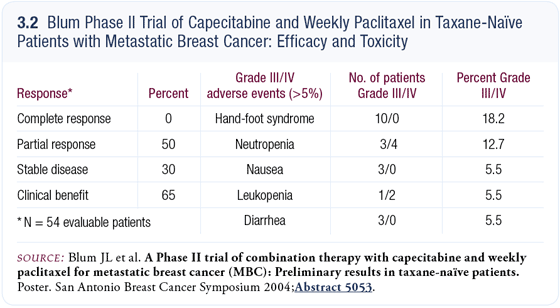
Joanne Blum evaluated another regimen of capecitabine with paclitaxel (Blum 2004) and demonstrated results similar to ours (3.2). Multiple studies have evaluated capecitabine plus a taxane. All of the studies are imperfect because none of them address the fundamental issue of whether one might accomplish the same objective with sequential, rather than combination, therapy. Studies are ongoing to address that issue.
Pivotal trial results of nanoparticle albumin-bound (nab) paclitaxel (Abraxane™)
The pivotal trial of patients with metastatic breast cancer compared paclitaxel 175 mg/m2 to nab paclitaxel 260 mg/m2 every three weeks. Patients receiving nab paclitaxel had a higher response rate — 30 percent versus 19 percent — and higher time to disease progression. Those findings were true in patients treated during first- and second-line therapy whether they had visceral disease or not (O’Shaughnesssy 2004). The results were also supported by an independent radiology review, which confirmed the findings throughout.
Nab paclitaxel also appears to be associated with far less severe myelosuppression and there is no need to administer steroids. One may have thought there would be no neuropathy with nab paclitaxel; however, it occurred in approximately 10 percent of patients. We’re also using a dose of paclitaxel in the nanoparticle formulation that’s 50 percent higher than standard paclitaxel — from 175 mg/m2 to 260 mg/m2.
Another interesting observation, corroborated in the pivotal trial and in the weekly trial that Joanne Blum reported (Blum 2003), is that the behavior of the neuropathy appears to be slightly different than that seen with standard paclitaxel. Although we don’t have sufficient data to be absolutely definitive, there is a suggestion that with nab paclitaxel the neuropathy is much shorter lived — on the order of 10 days to three weeks — and it tends to diminish to a point where you can re-treat the patients. That’s something that warrants further evaluation.
Physicians often ask how nab paclitaxel in this trial compares to docetaxel in a similar patient population. The first caveat is that we lack direct comparison data; however, if you evaluate the recently reported trials — including the comparison of paclitaxel to docetaxel in the Taxotere-311 study — the response rate with nab paclitaxel is strikingly similar to that of docetaxel at 100 mg/m2, with a similar time to disease progression. Prior trials of docetaxel had response rates similar to that of nab paclitaxel, so with all the limitations of comparing across trials, there’s at least a suggestion that the antitumor effect of nab paclitaxel is similar to docetaxel.
Optimizing adjuvant endocrine therapy
The ATAC trial adds to the existing body of evidence suggesting that the aromatase inhibitors incrementally improve outcome in patients who are postmenopausal with hormone-sensitive breast cancer. The results reported by Tony Howell at the 2004 San Antonio meeting suggest continued improvement in disease-free survival for patients receiving anastrozole compared to patients receiving tamoxifen, which simply confirms previous reports (Howell 2005).
Although we haven’t seen a survival benefit, we may still see one down the line, but this was a favorable risk group of patients. It reaffirms the idea that anastrozole would be a reasonable choice for newly diagnosed postmenopausal women with ER-positive disease. Another observation, true of all the other aromatase inhibitor trials, is that some degree of bone loss occurs.
We need to address that issue in the three aromatase inhibitors, but the degree of bone loss seems to be modest, and we have ways of addressing it in order to retain the positive aspects of the aromatase inhibitors. The trial data presented by Gnant demonstrated that the bone loss from goserelin and anastrozole could be eradicated by administering zoledronic acid twice per year, allowing us to think about optimizing adjuvant hormonal therapy without bone problems (Gnant 2004).
The ARNO study of anastrozole after two or three years of tamoxifen essentially replicates the IES trial with exemestane (Jakesz 2004). This trial evaluated whether five years of tamoxifen is optimal or whether an even greater benefit could be achieved by switching to anastrozole after two to three years of tamoxifen. This trial mirrors the report in the New England Journal of Medicine with exemestane, demonstrating an improved disease-free survival in patients who switched to exemestane (Coombes 2004).
I sit on the NCCN guidelines committee. If you evaluate the next rendition of the guidelines you’ll find we have not dismissed the use of tamoxifen but rather moved the use of aromatase inhibitors up front. Within the NCCN guidelines, we’re trying to select the aromatase inhibitor to be used based on the design of the study. For first-line therapy, we would use anastrozole. If a patient has been on tamoxifen for a period of time, exemestane is now a legitimate choice, and after five years of tamoxifen, letrozole is an option. We view all of these agents as active and well tolerated.
Evaluation of Fulvestrant versus Exemestane Trial (EFECT)
If you evaluate most of the available data with endocrine agents in the metastatic setting — tamoxifen, steroidal or nonsteroidal aromatase inhibitors or fulvestrant — the question that comes up is whether one sequence enhances patient outcome more than another. This becomes somewhat important, because if you could demonstrate that one sequence enhances the time to disease progression, it may be built on over time so that overall outcome is improved.
In theory, simply having an improvement in recurrence or progression of metastatic disease impacts quality of life. Patients now typically receive a nonsteroidal aromatase inhibitor — anastrozole or letrozole as the first treatment. The question became: If patients progress on one of those agents, what would be the next best therapy? Should it be the steroidal aromatase inhibitor exemestane or should it be fulvestrant?
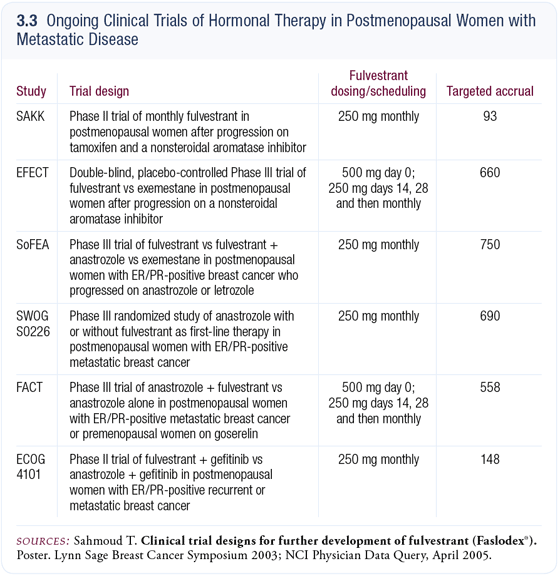
Indirect data evaluating the sequence of a nonsteroidal aromatase inhibitor to fulvestrant suggest that 25 to 30 percent of patients may receive some benefit with that approach. The question being asked by EFECT is whether patients who’ve received a nonsteroidal aromatase inhibitor (3.3) should receive fulvestrant or exemestane.
We are randomly assigning patients to evaluate the response rate and the duration of the time until disease progression. At present, EFECT is accruing both in Europe and in North America and is on target to finish in the next year or so.
An important issue is whether fulvestrant 250 milligrams is optimal, even though that’s the approved dose. Some of the data, including preclinical data generated by Kent Osborne and others, suggest that the dose is really on the low end of the curve where you might expect the optimal response rate. Although we may be able to increase the dose, giving 250 milligrams in each buttock, doing that too frequently becomes prohibitive, and patients may not tolerate it.
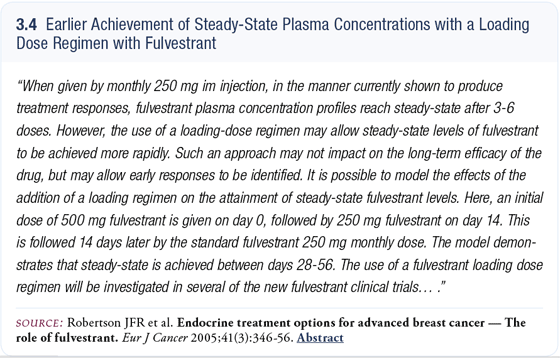
Some strategies have evaluated quickly increasing serum levels of fulvestrant and those strategies have included administering loading doses of 500 milligrams and then, within two weeks, administering another 250 milligrams and then proceeding to the monthly schedule.
Those strategies are based on mathematical modeling that have shown an ability to achieve steady-state levels much quicker and, consequently, achieve a biologically relevant dose of drug circulating in a given patient much faster (3.4). The EFECT trial has that strategy built into it with a 500-milligram loading dose.
Select publications
 |
Dr Gradishar is the Director of Breast Medical Oncology and Associate Professor of Medicine at the Robert H Lurie Comprehensive Cancer Center at Northwestern University Feinberg School of Medicine in Chicago, Illinois. |
|

