Michael Gnant, MD |
EDITED COMMENTS |
 Background to the study of aromatase inhibitors in premenopausal women Background to the study of aromatase inhibitors in premenopausal women
We are moving toward treating every patient with an aromatase inhibitor. While these drugs are more effective than tamoxifen and better tolerated in many ways, they have one clear-cut limitation — their effect on bone. They exert the positive effect of keeping the cancer away by reducing estrogen, but this is not good for bone density or bone quality.
We were particularly interested in younger patients because they are physiologically used to higher levels of estrogen from their functioning ovaries. We undertook ABCSG-12 to first establish the severity of that treatment-induced bone loss and, second, whether it can be prevented or treated (Gnant 2004).
We found out that a significant loss occurs — on average close to 15 percent — in these premenopausal women treated with endocrine therapy. We also discovered that it could be prevented with zoledronic acid given twice a year, which we believe is an elegant and easy way to eliminate the problem.
ABCSG-12: Study design
The ABCSG-12 study is a four-arm trial for premenopausal, hormone receptor-positive breast cancer patients (Gnant 2004; [4.1]). Patients in this trial are treated with endocrine treatment alone. They can receive preoperative chemotherapy to facilitate breast conservation, but postoperative chemotherapy is not administered because we have previously established that these patients — particularly the good-prognosis subgroup — can be treated without adjuvant chemotherapy.
All patients receive goserelin and are randomly assigned to receive tamoxifen versus anastrozole or the two treatments plus zoledronic acid. The treatment is for three years. This trial aims to establish the value of aromatase inhibitors for premenopausal patients because the results we have so far are derived from postmenopausal patients. We will recruit 1,800 patients and currently we have accrued close to 1,400.
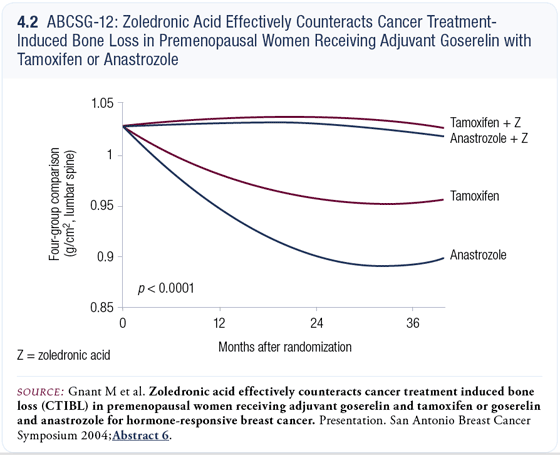
We currently have three-year results from the bone substudy, which closed 18 months ago with 401 patients. These patients received repeated DEXA measurements of their bone density in both their lumbar spine and trochanter.
Results of the bone subprotocol: Bone loss with tamoxifen
Our presentation two years ago was criticized because it was considered very early, despite the fact that this was the initial plan. Then the Data Monitoring and Steering committees decided the trial would have to be enlarged in order to prove that what we saw then was scientifically sound. Now, the results are beyond any doubt because we have much more mature data in the sense that most patients have their three-year measurements in (Gnant 2004). There will also be a five-year measurement.
One of the things that we see is some bone loss, even in the women on tamoxifen and goserelin. In postmenopausal women, we know that tamoxifen acts by basically protecting the bone with its estrogenic agonistic effects. In the premenopausal woman, however, tamoxifen is not able to balance the effects of ovarian suppression, so we see 11 percent bone loss with goserelin and tamoxifen (4.2).
Several ongoing prospective studies are monitoring bone density in postmenopausal women receiving an aromatase inhibitor — such as the Zometa-Femara® Adjuvant Synergy Trial (Z-FAST/ZO-FAST). The six-month results from the ZFAST trial show a three percent difference between an up-front prevention type approach with zoledronic acid versus waiting for bone loss in order to treat it (Brufsky 2004).
But bone loss is associated with age, and I believe it also makes a difference whether you start out with a vivid and functioning estrogen metabolism versus a 75-year-old lady in whom estrogen levels are, by nature, very low.
Approach to bone in postmenopausal women on aromatase inhibitors
We have implemented a general recommendation for postmenopausal women in our country to have annual measurements of bone mineral density. So far, this has not been done on a systematic basis. Once diagnosed, bone loss should be treated.
Treatment-induced bone loss should be treated the same way natural osteoporosis is treated. My suspicion is that oncologists have a tendency to overlook the problem. If nothing else, we can contribute to awareness: We should not underestimate the problem and should treat it accordingly.
In terms of deciding when to initiate therapy and what therapy to use, we follow the recommendations of the ASCO Bisphosphonate Panel (Hillner 2003) and the American Osteoporosis Society, which suggest that treatment with calcium/ vitamin D should be used for women with osteopenia — T scores between -1 and -2.5. We initiate bisphosphonates in women with osteoporosis where the T score goes down below -2.5.
Switching from tamoxifen to anastrozole after two years
Raimund Jakesz from our group presented a combined analysis of an Austrian and a German trial, which encompassed 3,200 patients overall (Jakesz 2004). This was a comparison of switching from tamoxifen to anastrozole after two years compared to keeping patients on tamoxifen for five years in the adjuvant, postmenopausal, receptor-positive setting.
This is a clean study in which 100 percent of patients are receptor-positive. A 40 percent reduction in risk of relapse occurred in patients who switched compared to patients maintained on tamoxifen. This meets our stopping boundaries and we are currently discussing how to deal with that.
Of course, we have to inform the patients, and we will either close the trial or at least amend it in some way. In terms of side effects and toxicity, we have observed that basically all the aromatase inhibitor trials have seen a benefit to aromatase inhibitors in terms of gynecological side effects, but again, with more fractures as compared to the tamoxifen group.
One specific point that is different from the other trials is that in our trial, switching is most effective in terms of preventing distant metastases. This is interesting, because although we do not have a good explanation, it suggests there might be a later survival benefit if we keep the trial alive and keep following the patients. It’s very exciting.
The effects observed are comparable in magnitude to those seen in the IES trial of switching to exemestane. You have to be quite cautious making indirect comparisons between trials, but I would suggest that the data are in the same direction. I was personally hoping that exemestane would be a little different in terms of bone, because of its steroidal structure, but this does not appear to be the case.
Select publications
 |
Dr Gnant is a Professor of Experimental Surgical Oncology at the Medical University of Vienna in Vienna, Austria. |
|

