Case history:
• December 2004
Mrs B is a 57-year-old woman who was diagnosed with ER-positive invasive breast cancer in April 2001. She had one positive sentinel lymph node and received AC T chemotherapy, after which her oncologist prescribed tamoxifen. The patient and the doctor discussed the recently presented ATAC data; however, the oncologist recommended that they “stick with the tried and true endocrine therapy.” T chemotherapy, after which her oncologist prescribed tamoxifen. The patient and the doctor discussed the recently presented ATAC data; however, the oncologist recommended that they “stick with the tried and true endocrine therapy.”
Mrs B has now been receiving tamoxifen for two and a half years. Her bone density is normal, and she has no complaints other than modest weight gain and vasomotor symptoms that she attributes to tamoxifen. Since the original diagnosis, at least five major randomized trials have demonstrated that patients treated with aromatase inhibitors experienced fewer relapses compared to patients receiving tamoxifen either in the up-front adjuvant setting or after two to three or five or more years of tamoxifen.
During a routine follow-up visit, the oncologist mentions these studies but recommends continuing tamoxifen. The patient agrees.
• October 2005
Mrs B is seen for an unscheduled visit because of the gradual and progressive onset of lower back pain. A diagnostic workup reveals bone and pulmonary lesions compatible with metastases, and needle biopsy of the lung confirms recurrence. An aromatase inhibitor is initiated. |
Our CME group recently published a first ever “Patterns of Care” case-based survey of national breast cancer clinical research leaders. The fascinating results from this project were then compared to a previous identical survey of community-based oncologists.
One of the most important findings from this comparison is the suggestion that the case scenario described above is happening every day in this country, mainly in community practice as opposed to academic centers (1.1).
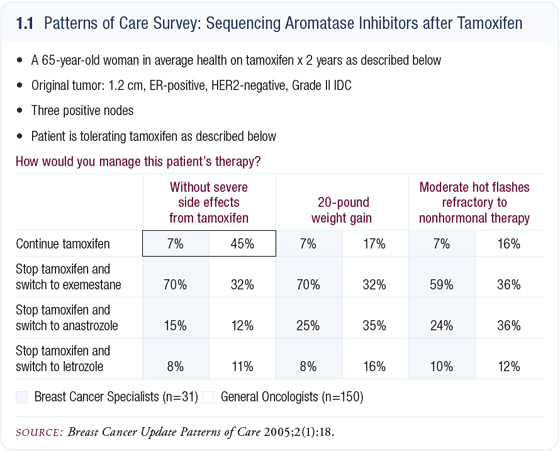
The debate over the role of aromatase inhibitors versus tamoxifen in the adjuvant setting continues, and counter-arguments can be made on each side. For every patient who develops a fracture on an aromatase inhibitor, other patients experience a DVT, stroke or endometrial cancer on tamoxifen; however, for most people with breast cancer, the overwhelming concern is decreasing the likelihood of disease recurrence. At this point, aromatase inhibitors clearly do it better.
One might “cover oneself” ethically and perhaps legally by sharing with patients what we know about the risks and benefits of various options for longterm adjuvant endocrine therapy, but patients also want a recommendation. It is remarkable that postmenopausal patients visiting breast cancer specialists today are much more likely to be encouraged to switch to an aromatase inhibitor during their first five years of tamoxifen.
Gabe Hortobagyi has the most direct approach to this issue: He simply switches postmenopausal women to an aromatase inhibitor regardless of how long they have been on tamoxifen. Plain and simple, the elegance of this strategy is attractive, but currently it would have to be labeled “C” for controversial.
In this edition of our series, Tony Howell and Michael Gnant, the PI and co-PI of two critical Austrian trials of endocrine therapy, update us on the rapidly evolving clinical trial results with the aromatase inhibitors in the adjuvant setting.
Tony eloquently reviews not only the 68-month update from ATAC but also the first results of the BIG 1-98 trial with data on letrozole versus tamoxifen at 30 months. While the efficacy findings of these two trials look similar at early time points, Tony notes the unexpected finding of increased deaths from myocardial infarction in patients treated with letrozole in the BIG study. He goes on to speculate about whether continued follow-up of trials of all three major aromatase inhibitors will show differences in their safety profiles, particularly related to cardiovascular disease.
At this point, community-based and research oncologists are generally starting postmenopausal patients with ER-positive tumors on an aromatase inhibitor — usually anastrozole. One of the major reasons oncologists have a greater comfort level with this treatment strategy is their increasing confidence with regard to the issue of bone loss. In great part, this can be attributed to Dr Gnant’s work evaluating zoledronate in premenopausal patients made postmenopausal with an LHRH agonist and then treated with either tamoxifen or anastrozole.
I interviewed Dr Gnant at the 2002 San Antonio Breast Cancer Symposium when he presented the first data set from his trial demonstrating that zoledronate totally abrogated bone loss in both patient populations. This encouraging finding was unchanged with two more years of follow-up in his most recent presentation at the 2004 San Antonio meeting and is good news, as are the ATAC observations that there is no increase in the rate of hip fractures, and that the overall fracture rate is decreasing after patients stop anastrozole at five years. Nonetheless, the bone issue must continue to be closely monitored and Dr Gnant puts a plug in for good old-fashioned outdoor exercise as a means to improve bone density. He gently chides American women who “don’t go out in the winter because it’s too cold, and don’t go out in the summer because it’s too hot.” I guess they don’t have TiVo® in Austria.
Dr Gnant also discusses findings from another important endocrine trial, specifically the Austrian/German study that randomly assigned patients at two to three years to either continue tamoxifen or switch to anastrozole. As with the other large switching trial — the IES study with exemestane — these data documented a major reduction in relapse rate in patients who switched to anastrozole, and most of the events avoided were distant recurrences. These findings directly relate to the patient presented at the beginning of this commentary, and one can say with reasonable confidence that there is at least a one-in-three chance that this woman would have remained recurrence free if she had switched to exemestane or anastrozole.
The same can be said about letrozole with regard to the patient who completes five years of tamoxifen. Peter Ravdin’s Adjuvant! model* now provides estimates of risk of relapse at various time points after diagnosis and how these might be modified by the use of an aromatase inhibitor. This information should be offered to all postmenopausal women on adjuvant tamoxifen.
* www.adjuvantonline.com
The decision to “switch or not switch” will probably only be on the table for the next few years, as the last remaining patients from the “pre-acceptance of ATAC” era pass through oncology offices. Nonetheless, tens of thousands of people with breast cancer are currently receiving therapy that appears to have a suboptimal risk-to-benefit ratio, particularly related to breast cancer control. All of our adjuvant systemic interventions improve the odds for patient populations globally, but for an individual person, we never know the exact impact of a specific therapy. When relapse does occur, both patient and physician look back and hope that prior decisions about adjuvant therapy offered the best chance to remain recurrence free.
In that regard, one of the common explanations patients and oncologists provide when justifying their decision to begin adjuvant chemotherapy for small nodenegative tumors is the need to feel that they are doing everything possible to prevent disease recurrence. This does a great deal to prevent painful feelings of regret if relapse occurs, and one might assume that this same thought would and should apply to a significantly less toxic intervention like endocrine therapy.
Someone (ASCO Tech Assessment #4?) needs to step up to the plate and make it clear that at this point, five years of adjuvant tamoxifen is suboptimal adjuvant therapy for many or most postmenopausal patients with ER-positive invasive breast cancer.
—Neil Love, MD
NLove@ResearchToPractice.net
Select publications
BIG 1-98 Collaborative Group. Letrozole vs tamoxifen as adjuvant endocrine therapy for postmenopausal women with receptor-positive breast cancer. BIG 1-98: A prospective randomized double-blind Phase III study. www.ibcsg.org. Abstract
Coombes RC et al; Intergroup Exemestane Study. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 2004;350(11):1081-92. Abstract
Gnant M et al. Zoledronic acid effectively counteracts cancer treatment induced bone loss (CTIBL) in premenopausal breast cancer patients receiving adjuvant endocrine treatment with goserelin plus anastrozole versus goserelin plus tamoxifen — Bone density subprotocol results of a randomized multicenter trial (ABCSG-12). San Antonio Breast Cancer Symposium 2004;Abstract 6.
Goss PE et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 2003;349(19):1793-802. Abstract
Howell A et al; ATAC Trialists’ Group. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 2005;365(9453):60-2. Abstract
Jakesz R, on behalf of the ABCSG. Benefits of switching postmenopausal women with hormone sensitive early breast cancer to anastrozole after 2 years adjuvant tamoxifen: Combined results from 3,123 women enrolled in the ABCSG Trial 8 and the ARNO 95 Trial. San Antonio Breast Cancer Symposium 2004;Abstract 2.
Winer EP et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptorpositive breast cancer: Status report 2004. J Clin Oncol 2005;23(3):619-29. Abstract
|

