Anthony Howell, MD |
EDITED COMMENTS |
 ATAC trial: 68-month follow-up ATAC trial: 68-month follow-up
Disease-free survival and overall survival
We now have the 68-month data from the ATAC trial, and we see continued improvement in disease-free survival in patients receiving anastrozole versus tamoxifen — a 3.3 percent absolute difference and a 17 percent improvement in the hazard ratio for relapse in hormone receptor-positive patients (Howell 2005).
Anastrozole improves the recurrence rate and the time to distant recurrence; we also saw a nonsignificant improvement in time to breast cancer death. However, no difference in overall survival has yet been demonstrated between patients receiving anastrozole versus tamoxifen.
While we hoped to see a difference in mortality at this point, a mortality improvement was not observed with tamoxifen in NSABP-B-14 until after approximately seven years of follow-up. We’re at six years with ATAC, so it may be a year or two before any mortality improvement is demonstrated, but we do expect it to occur because we see a distant disease-free survival advantage with anastrozole.
Two-year recurrence rate and contralateral breast cancer
A peak in recurrences occurs at two years for patients on tamoxifen, and it’s similar to the peak that we see in patients who receive no treatment. We see this peak in all patients on tamoxifen but especially in patients who have nodepositive disease. This two-year peak was blunted by anastrozole.
This is obviously important because if patients start with tamoxifen, some will relapse on tamoxifen who would not have relapsed on anastrozole, and we’ve lost those patients.
In addition, we see increased toxicity with tamoxifen over those first two and a half years, so from both the efficacy and toxicity standpoints, it is probably better to begin adjuvant hormonal therapy with an aromatase inhibitor.
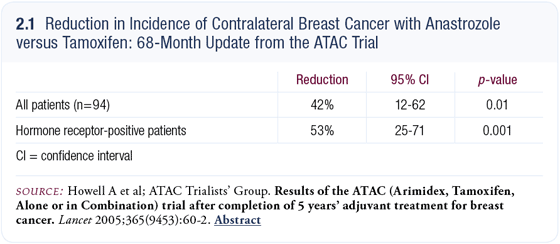
Contralateral breast cancer rate and prevention
In the ATAC trial, contralateral breast cancer was reduced by 50 percent with anastrozole (2.1), which is similar to the data from other aromatase inhibitor trials. That’s a 50 percent reduction compared to tamoxifen, but it’s a 75 percent reduction compared to no treatment.
The ATAC data prompted investigators to launch the IBIS-2 prevention trial in which patients at increased risk are randomly assigned to anastrozole versus placebo. We also have an IBIS-2 trial for patients with DCIS in which the randomization is the same as in the NSABP DCIS trial — tamoxifen versus anastrozole. In our DCIS trial, we are comparing anastrozole to tamoxifen because that is the standard; however, we are using a placebo in the high-risk trial because we don’t believe tamoxifen is the standard for patients who are at increased risk for breast cancer but do not have DCIS.
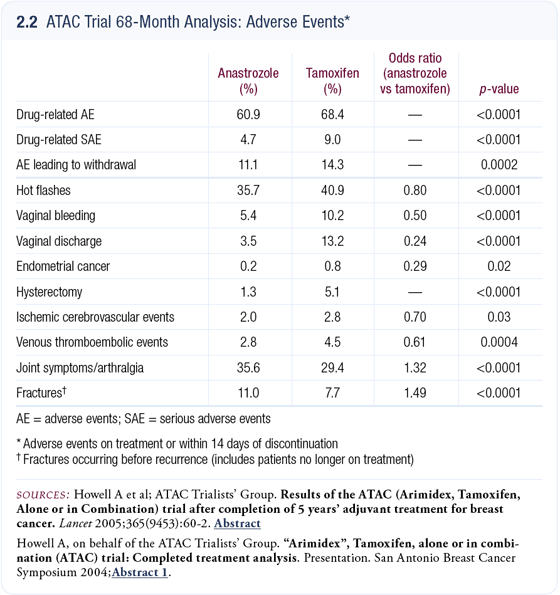
ATAC toxicity data
At the 2004 San Antonio meeting, we presented updated toxicity data including new data on hysterectomy rates (2.2). The rate on tamoxifen was 5.1 percent, whereas on anastrozole it was only 1.3 percent (Howell 2004). The rate of endometrial cancer is 0.8 percent on tamoxifen and 0.2 percent on anastrozole, so clearly endometrial cancer doesn’t account for all of the increase seen in the hysterectomy rate. This suggests that some women are undergoing unnecessary hysterectomies. I believe this issue pushes the economics in favor of anastrozole despite the increased cost of this agent.
The other story is the joint symptoms we see with aromatase inhibitors. In the data reported, tamoxifen had approximately a 29 percent joint symptom rate and with anastrozole the rate was approximately 36 percent. Matt Ellis’ group presented an interesting abstract at San Antonio indicating that women with these symptoms may have lowered vitamin D levels and that giving them vitamin D improved some of the joint symptoms (Taylor 2004). The data are very early, and they are conducting more studies, but if we could solve this joint problem with vitamin D, it would be extraordinary.
We know from the ATAC trial that more serious adverse events are associated with tamoxifen than with anastrozole, and that despite the joint symptoms, patients tend to stay on anastrozole more than they stay on tamoxifen, which is an important efficacy issue.
Bone density
In the 68-month follow-up of the ATAC trial, the fracture rates were 7.7 percent with tamoxifen versus 11 percent with anastrozole (Howell 2005). We saw no increase in hip fractures with anastrozole, which is important, but the fracture rate with anastrozole is still a concern. Another issue is fracture rate over time, and I presented the data out to six years.
With tamoxifen, the annual fracture rate is approximately 1.5 to two percent, whereas with anastrozole it’s approximately 2.5 percent. What surprised me was that at year five-six in the trial, the fracture rate was lower in the anastrozole group than in the tamoxifen group, although not significantly lower. It seems that as soon as anastrozole is stopped, the fracture rate goes down.
The trial had a bone subprotocol in which we evaluated lumbar spine and trochanter bone mineral density over time. In the first year an approximately 2.5 percent drop in bone mineral density occurred on anastrozole, and at two years it was just over a four percent drop.
This is similar to the IES data with exemestane and the MA17 data with letrozole. The impact on bone mineral density is a class effect of aromatase inhibitors and is not limited to anastrozole. We need to learn how to manage this (Coleman 2004; Perez 2004).
In San Antonio, Michael Gnant presented the extraordinary Austrian data on using zoledronic acid to prevent bone mineral loss in premenopausal patients (Gnant 2004). Patients were randomly assigned to receive goserelin plus tamoxifen versus goserelin plus anastrozole; then, in a subrandomization, patients received zoledronic acid or not. They found that the loss of bone mineral density on anastrozole over three years in this study was completely abrogated by administering zoledronic acid.
Deep vein thrombosis, stroke and cardiovascular events
In the ATAC trial, 4.5 percent of women on tamoxifen experienced deep vein thrombosis, whereas approximately 2.8 percent on anastrozole developed this side effect. That’s similar to the other studies and similar to the rate seen in women on hormone replacement therapy. We also continue to see a reduction in ischemic cerebrovascular events on anastrozole versus tamoxifen — two percent versus 2.8 percent, respectively.
The important data in my mind are the new and slightly worrisome findings on cardiac events in the aromatase inhibitor trials. In ATAC, the rate of cardiac events was 4.1 percent on anastrozole and 3.4 percent on tamoxifen. The increase on anastrozole was not statistically significant — the p-value was 0.12.
The IBCSG-1-98 data presented at the St Gallen’s meeting reported on increases in Grades III to V cardiac events with letrozole, which were statistically significant. The rates were 3.6 percent in patients on letrozole compared to 2.5 percent in patients on tamoxifen, with 26 versus 13 myocardial deaths, respectively (BIG 1-98 Collaborative Group 2004).
Coombes, reporting on the IES trial in San Antonio, reported a statistically significant increase — 20 myocardial infarctions on exemestane and eight on tamoxifen (Coombes 2004). This issue needs to be monitored carefully.
IBCSG-1-98: Letrozole versus tamoxifen up front or sequentially
The IBCSG-1-98 trial has approximately 4,000 patients in each of four arms. Patients in the first arm receive tamoxifen for five years. In the second arm, patients begin with tamoxifen and then switch to letrozole. In the third arm, patients begin on letrozole and switch to tamoxifen, while patients in the fourth arm receive letrozole for five years.
The IBCSG-1-98 efficacy data at 30 months look almost identical to the ATAC data at 33 months, favoring the aromatase inhibitor over tamoxifen (BIG 1-98 Collaborative Group 2004; [2.3]). The disease-free survival is a 21 percent reduction in the IBCSG-1-98 versus a 22 percent reduction in the ATAC trial. Time to recurrence was reduced 18 percent in IBCSG-1-98 and 17 percent in ATAC. The distant disease-free survival, which is possibly a surrogate for breast cancer survival, is also similar to ATAC.
Selection of an aromatase inhibitor for adjuvant therapy
We have two studies evaluating an aromatase inhibitor up front — ATAC with anastrozole and IBCSG-1-98 with letrozole; however, we have more data on anastrozole with more than five years of follow-up. There doesn’t appear to be any difference in efficacy of these two agents, so thus far, I use anastrozole based on the toxicity profiles and longer follow-up.
I use exemestane after two to three years of tamoxifen based on the IES data (Coombes 2004). However, if you compare the IES exemestane data to the data from the combined ARNO 95/ABCSG-8 trials, in which the patients were switched to anastrozole, the agents appear to be similar in terms of efficacy (Jakesz 2004).
The hazard ratio for relapse-free survival was 0.73 in the IES study and 0.60 in the ARNO study, so I believe these two agents are equivalent in this situation. We now have data to support the use of either anastrozole or exemestane after two or three years of tamoxifen. After five years of tamoxifen, we have only the MA17 trial data, so I use letrozole in this setting (Goss 2003).
Adjuvant aromatase inhibitors in premenopausal patients with ER-positive breast cancer
Three important randomized trials are enrolling premenopausal women with hormone-receptive disease — SOFT, TEXT and PERCHE. The ABCSG-AU12 trial randomly assigned approximately 2,000 patients to goserelin plus tamoxifen versus goserelin plus anastrozole, with a second randomization to zoledronic acid or not. That study will report in one or two years and should tell us whether tamoxifen or an aromatase inhibitor is superior when combined with goserelin in premenopausal women. We expect that goserelin with anastrozole will be better, which is why so many patients are already being treated off protocol.
Aromatase inhibitors are ineffective in premenopausal women without ovarian suppression. We need to be careful when patients experience chemotherapyinduced amenorrhea. Some women will begin menstruating again, and estradiol levels can recover without menses. If you measure the estradiol level and it’s postmenopausal, it can go up again the next week and the aromatase inhibitor becomes ineffective. We know estrogen levels fluctuate in these women and we need to be absolutely certain the ovaries are suppressed, by using goserelin, for example, or even going so far as ovarian ablation.
Select publications
 |
Dr Howell is a Professor of Medical Oncology at the University of Manchester in Manchester, England. |
|

