Joyce O’Shaughnessy, MD |
EDITED COMMENTS |
 Phase II trial of capecitabine and paclitaxel Phase II trial of capecitabine and paclitaxel
We conducted this clinical trial in two different cohorts of about 50 patients with metastatic disease: taxane naïve and taxane pretreated.
If you’re going to administer capecitabine with any other agent (eg, paclitaxel, docetaxel or vinorelbine) in the adjuvant, neoadjuvant or metastatic setting, a dose of 1,650 mg/m2 per day seems to be well tolerated.
On a 21-day cycle, we administered paclitaxel 80 mg/m2 on days one and eight and capecitabine 1,650 mg/m2 per day in two divided doses, 14 days on and seven days off (Blum 2004).
The data from the taxane-naïve patients with metastatic breast cancer demonstrated a response rate of about 50 percent (2.1), and the toxicity was mild (Blum 2004). It was an easy clinical trial to conduct because many of us were already utilizing the combination of capecitabine and paclitaxel in our practices; however, we didn’t have any data for weekly paclitaxel and capecitabine.
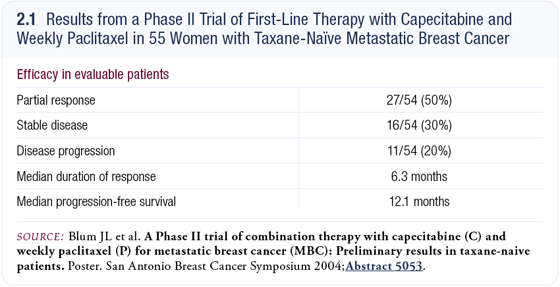
This regimen was extremely well tolerated. Some side effects were associated with capecitabine, and about one fourth of the patients required a dose reduction. I particularly like combinations like this that are well tolerated and allow us to treat patients for long periods of time. I think capecitabine/paclitaxel is a good regimen; it’s active and has manageable toxicity.
Dr Gradishar also reported in the Journal of Clinical Oncology on a regimen of capecitabine and every three-week paclitaxel with a response rate of 52 percent (Gradishar 2004). Of course, more myelosuppression occurs with paclitaxel administered at 175 mg/m2 every three weeks per day, but it is a well-tolerated regimen that has efficacy similar to our paclitaxel/capecitabine regimen.
Like all combination chemotherapies, fatigue occurs over time; however, many patients can continue for long treatment periods. I often stop the intravenous part of the regimen — in this case, paclitaxel — after six or eight cycles and continue with capecitabine alone.
Comparing capecitabine/docetaxel and capecitabine/paclitaxel
These two regimens have similar efficacy. The response rates and percentage of patients with prolonged stable disease are similar. With regard to toxicity, I think every three-week docetaxel is similar to every three-week paclitaxel — both cause more myelosuppression than weekly paclitaxel.
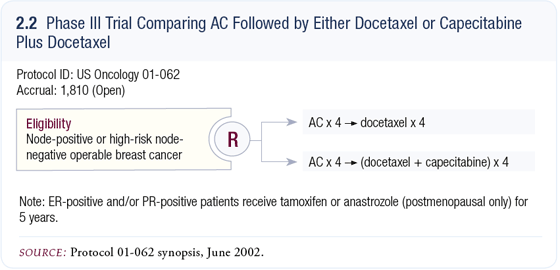
Patients develop a bit more asthenia with docetaxel. With capecitabine/docetaxel, lifting off of the nail beds is a prominent but reversible toxicity. In our adjuvant trial comparing AC followed by docetaxel to AC followed by capecitabine/ docetaxel (2.2), the nail toxicities are more common with the combination of capecitabine/docetaxel.
Additionally, docetaxel sometimes causes epiphora, which is not observed with weekly paclitaxel. With just four cycles of docetaxel or capecitabine/docetaxel in the adjuvant setting, the epiphora, which is fairly ubiquitous, is almost always completely reversible. In the metastatic setting, where patients receive more cycles of docetaxel, the epiphora may not be reversible without stenting.
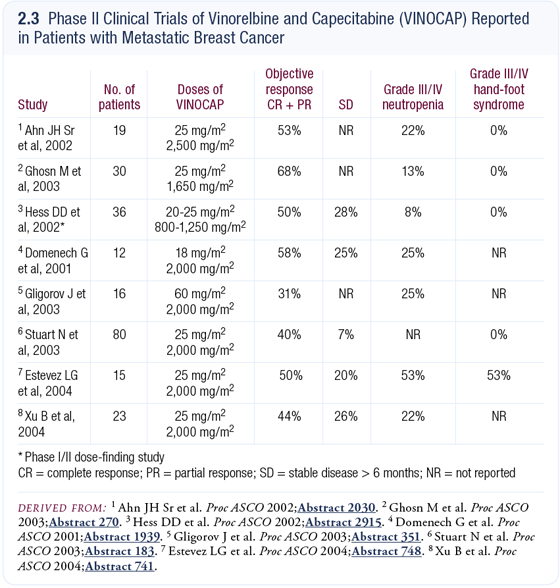
The VINOCAP regimen (vinorelbine/capecitabine)
I’ve used capecitabine in combination with vinorelbine administered on a day one and day eight schedule. VINOCAP does not cause alopecia, and Phase II trial data with this regimen indicate response rates in the 40 percent to 60 percent range (2.3). With that regimen, I stop the vinorelbine after a while and keep using capecitabine alone. That is a bit of a gamble because you don’t know if the woman is responding to one or the other or both agents. It’s rather imprecise but I think we have to make decisions based on toxicity.
Adjuvant clinical trials incorporating capecitabine
The vinorelbine/capecitabine combination is one of numerous capecitabine combinations being evaluated in European adjuvant trials. I’m not aware of any adjuvant or neoadjuvant studies evaluating capecitabine/paclitaxel; however, a number of neoadjuvant and adjuvant trials are evaluating capecitabine/ docetaxel.
Even if I had data with capecitabine/paclitaxel, I probably would not have considered evaluating that combination — as opposed to capecitabine/docetaxel — in our adjuvant trial. In metastatic disease, docetaxel 75 mg/m2 in combination with capecitabine has a clear survival advantage compared to docetaxel 100 mg/m2 (O’Shaughnessy 2002). Usually, we try to take that advantage in survival in metastatic disease and immediately move it into the adjuvant setting.
US Oncology neoadjuvant trial of FEC 100 followed by capecitabine/docetaxel
In women with T2, T3 or T4 clinical breast cancer who have been diagnosed by a core biopsy, we’re treating the patients preoperatively with four cycles of FEC 100 followed by four cycles of capecitabine in combination with weekly docetaxel 35 mg/m2 on day one and day eight. Then, the patients undergo surgery. Pretreatment tumor specimens are sent to Dr Lajos Pusztai at MD Anderson for microarray analysis to predict who’s going to have a pathologic complete response (pCR).
Since Dr Aman Buzdar presented the exciting data from MD Anderson at ASCO 2004 — indicating a 67 percent pCR rate with FEC, paclitaxel and trastuzumab (Buzdar 2004) — we have been working hard to expand our current trial by adding an additional cohort of patients with HER2-positive disease. We will still use FEC followed by capecitabine/docetaxel but, like Dr Buzdar, we’ll drop the epirubicin dose to 75 mg/m2 and add trastuzumab. We will see if we can reproduce his high pCR rate and obtain additional cardiac safety data.
I usually use AC followed by docetaxel in the preoperative setting but I am impressed with FEC 100, which is very effective in treating primary breast lesions. My colleagues in US Oncology who have been using FEC 100 preoperatively say it is highly effective, and I’ve recently seen that for myself. FEC followed by capecitabine/docetaxel results in a fair number of pCRs.
Trastuzumab in the neoadjuvant and adjuvant settings
From the cardiac safety perspective, I think it’s a bit soon to utilize Dr Buzdar’s neoadjuvant trastuzumab regimen in a nonprotocol setting. Although he has accrued additional patients and the cardiac safety is holding up, I think we need more data.
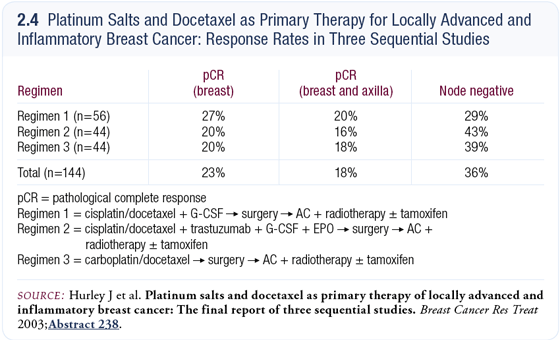
Mark Pegram has data with a preoperative regimen of docetaxel, carboplatin and trastuzumab (TCH; [2.4]), which is showing a pCR rate in the same range as that seen by Judith Hurley with a similar regimen (Hurley 2003). We do not yet have Phase III data with regard to safety and efficacy, but I think it’s beginning to emerge as a reasonable option.
I tend to treat women with locally advanced disease preoperatively without trastuzumab. If they don’t have a pCR after surgery, then I start trastuzumab. For example, I might use preoperative FEC or CAF for four cycles, send the patient to surgery and evaluate the antitumor response. If the woman still has a lot of cancer in her lymph nodes or breast and has strongly HER2-positive and ER/PR-negative disease, then I’ll treat her with four cycles of TCH afterward. In women with inflammatory breast cancer, I use a similar approach — preoperative CAF or FEC, surgery and then TCH.
I’ve done this judiciously and only in patients with the highest-risk disease. The NSABP-B-31 cardiac safety data (Geyer 2003) allows us to provide information about the cardiac risks associated with a taxane and trastuzumab following four cycles of doxorubicin. I administer four cycles of TCH, then stop the chemotherapy and continue trastuzumab. I switch the trastuzumab to an every three-week regimen and continue it for one year.
Synergy between the anthracyclines and trastuzumab
From a molecular standpoint, about 40 percent to 50 percent of patients with HER2 overexpression will have topoisomerase II alpha (topo-II) gene amplification, which increases sensitivity to the anthracycline. Most HER2-driven breast tumors are highly proliferative. Even if they don’t have topo-II gene amplification, they have a lot of protein because they’re so highly proliferative. Doxorubicin targets these highly proliferative cells. Adding trastuzumab creates a highly synergistic combination.
In the pivotal trial by Dr Slamon, a regimen of an anthracycline and cyclophosphamide with trastuzumab was highly effective but was associated with significant cardiac toxicity. The survival advantage associated with the addition of trastuzumab was higher with an anthracycline and cyclophosphamide than with paclitaxel (Slamon 2001).
Interestingly, a lot of work is ongoing with epirubicin and trastuzumab. Dr PierFranco Conte is conducting a trial in Italy that combines FEC with trastuzumab as either adjuvant or neoadjuvant therapy. The German groups are evaluating EC for four cycles with trastuzumab, and they’re doing quite well.
The Europeans, however, are utilizing 90 mg/m2 of epirubicin with four cycles of trastuzumab, and they’re not running into cardiac problems. It’s encouraging.
Select publications
 |
Dr O’Shaughnessy is Co-Director of the Breast Cancer Research Program at Baylor-Charles A Sammons Cancer Center, US Oncology in Dallas, Texas. |
|

