Raimund V Jakesz, MD |
EDITED COMMENTS |
 Rationale for sequencing endocrine therapies in the adjuvant setting Rationale for sequencing endocrine therapies in the adjuvant setting
When a patient experiences resistance to one endocrine agent, that doesn’t mean the cancer has become endocrine resistant. We know from the metastatic setting that a hormone-responsive tumor that responds to tamoxifen, but then progresses a year later, has a high likelihood that it will respond to another endocrine agent and again to third- and fourth-line endocrine treatments.
The tumor may become resistant to one drug, but we do not abolish the tumor’s hormone dependency. We are now transferring that knowledge gained in the metastatic palliative setting to the adjuvant setting by evaluating trials of switching endocrine agents.
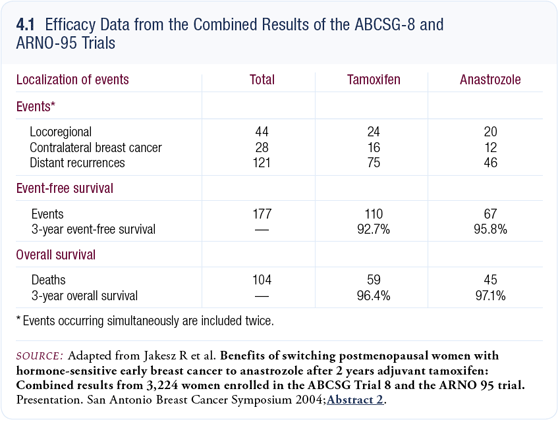
ABCSG-8 and ARNO-95: Switching to anastrozole after two years of adjuvant tamoxifen
In the combined trials of ABCSG-8 and ARNO-95, more than 3,200 postmenopausal patients, all with receptor-positive disease, were exposed to two years of adjuvant tamoxifen after primary surgery. We then randomly assigned them to tamoxifen or anastrozole for three years. The tumors were generally moderately well differentiated, and 95 percent were T1 or T2 lesions, 75 percent were node negative, and none of the patients received chemotherapy. It was clean, informative data.
With a median follow-up of 28 months, we found that switching to anastrozole reduced the likelihood of developing an event by 40 percent, which was highly significant (Jakesz 2004; [4.1]).
Most of the difference seen in event rate with anastrozole was due to a huge reduction in distant metastases. In the group treated with tamoxifen for five years, 75 patients developed distant metastases, whereas only 46 patients did so in the sequenced group. Perhaps in two or three years, this might translate to an improvement in overall survival.
ABCSG-8/ARNO-95: Safety data
Anastrozole is well tolerated and no treatment-related deaths occurred in these trials. Anastrozole did not cause an increase in cardiovascular disease or pulmonary disease, but a significant increase in fractures occurred. The fracture rate in the anastrozole group was 2.4 percent versus 1.2 percent in patients who received tamoxifen (Jakesz 2004). That’s much lower than what we’ve seen in the ATAC trial, but that’s because all the patients in our study were initially treated with tamoxifen, which, due to its partial agonistic effect, protects bone.
We didn’t see many gynecological side effects probably because we counted side effects only after randomization. In patients on tamoxifen, gynecological side effects usually start in the first two years.
Switching from tamoxifen to either exemestane or anastrozole
ABCSG-8 and ARNO-95 — utilizing anastrozole — serve as confirmatory trials for the IES study, which used exemestane. I believe anastrozole and exemestane are similar in efficacy but have a different safety profile.
In the IES trial, exemestane resulted in a risk reduction of approximately 35 percent (Coombes 2004), whereas in the combined trials the risk of an event was reduced by 40 percent with anastrozole.
It was hoped that exemestane would have a protective effect on bone, but that is obviously not true.
ATAC trial: 68-month efficacy and safety data
The 68-month follow-up of the ATAC trial was presented at the San Antonio Breast Cancer Symposium and also recently published in The Lancet (Howell 2004, Howell 2005). An impressive trend for the reduction in the cancer-specific recurrences is seen with anastrozole, and the five-year recurrence-free survival differed by 3.3 percent. A carryover effect obviously exists and the curves diverge, which is a nice result.
On the other hand, the lack of improvement in overall survival is important. The ATAC trial was not as clean as the ABCSG-8 and ARNO-95 trials in that the ATAC study included patients with estrogen receptor-negative tumors and patients who received chemotherapy.
The safety profile in the update still favors anastrozole. The incidence of endometrial cancer is 0.2 percent with anastrozole and 0.8 percent with tamoxifen. The new data revealed a 5.1 percent rate of hysterectomy with tamoxifen and only slightly over one percent with anastrozole. Also, with anastrozole we seldom see gynecological side effects, such as bleeding or discharge, and we see no increased risk of strokes or pulmonary embolism.
Switching endocrine therapies to avoid subclinical resistance
Anastrozole is certainly more potent than tamoxifen, and it significantly reduces the incidence of contralateral breast cancer; however, we don’t know the best sequence for the various endocrine agents. We need more sequencing trials. I believe the longer a tumor is exposed to a specific drug, the more likely it will develop subclinical resistance and eventually metastasize.
ABCSG-12: Zoledronic acid
ABCSG-12 is an adjuvant trial comparing goserelin plus tamoxifen to goserelin plus anastrozole in premenopausal patients with ER-positive disease. It’s similar to the ATAC trial but studies premenopausal patients. We were concerned about the impairment of the bone mineral density, so both groups are further randomized to receive zoledronic acid or not. We have recruited approximately 1,400 patients and have approximately 1,200 bone mineral density measurements.
The trial is ongoing and we need to accrue approximately 400 more patients. Although we don’t know the mechanism, it’s well known that tamoxifen causes bone loss in premenopausal women, whereas it strengthens bone in postmenopausal women. As expected, patients on goserelin/anastrozole have a higher reduction in bone mineral density in the lumbar spine than patients receiving the goserelin/tamoxifen combination — approximately a 17 percent versus 11 percent reduction, respectively (Gnant 2004); however, we have seen that the bone loss for both groups can be entirely prevented by the administration of zoledronic acid.
This is a remarkable trial. I don’t know what we will see with long-term follow-up, but I hope we can further improve the prognosis for these patients by administering anastrozole instead of tamoxifen. We are continuing to randomly assign patients to the arms without zoledronic acid, but every other year we perform a bone mineral density measurement and treat patients according the ASCO guidelines as advised by an independent data monitoring committee. Whether zoledronic acid has an oncological benefit, we don’t know yet, but I believe this is likely — and that would be a landmark finding.
Anastrozole following five years of adjuvant tamoxifen
We have submitted an abstract to the 2005 ASCO meeting and hope to present data from a trial in which, after five years of adjuvant tamoxifen, patients were randomly assigned to three years of anastrozole versus no further treatment. In the MA17 trial, patients received letrozole for five years after tamoxifen, but in our trial the anastrozole exposure was only three years. The results are important and are still confidential at this time. Currently, I discuss the MA17 data with patients and recommend that they take letrozole for at least two or three years after tamoxifen.
Estrogen receptor status and response to chemotherapy in postmenopausal patients
In estrogen receptor-negative tumors, we use chemotherapy in all patients with lesions greater than one centimeter; however, in estrogen receptor-positive tumors, we use chemotherapy only in high-risk cases such as undifferentiated, HER2-overexpressing tumors with five or more positive nodes.
It is important to separate estrogen receptor-positive and receptor-negative tumors when considering chemotherapy and when conducting clinical trials. To lump all these patients together doesn’t reflect the biology of the tumor. These are different types of cancer. The patients should be treated differently and studied separately.
I believe that postmenopausal patients do not respond as well to chemotherapy and that receptor status affects response. Tumors proliferate more slowly in patients with estrogen receptor-positive disease; however, this is not well studied. We conducted a retrospective analysis of 250 patients who received preoperative chemotherapy, and we found no cases of pCR in tumors that were estrogen and progesterone receptor-positive.
Select publications
 |
Dr Jakesz is a member of the Department of Surgery at the Vienna Medical School and President of the Austrian Breast and Colorectal Cancer Study Group in Vienna, Austria. |
|

