Charles E Geyer Jr, MD |
EDITED COMMENTS |
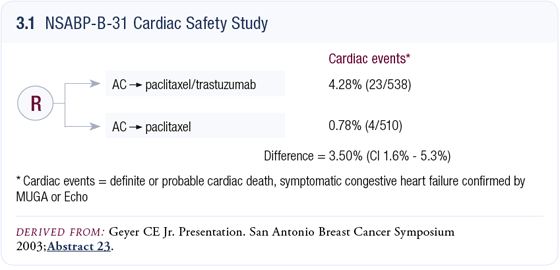
 Cardiotoxicity in the NSABP trial B-31 evaluating adjuvant trastuzumab Cardiotoxicity in the NSABP trial B-31 evaluating adjuvant trastuzumab
In the cardiac safety study, we waited until we had the 18-month follow-up on most patients because recoverability is clearly an important issue (Geyer 2003). Certainly, we need to identify the rates and severity of toxicity, but with the appreciation that the cardiotoxicity is, to a large degree, reversible.
In patients receiving trastuzumab, we continue to have approximately a four and a half percent incidence of symptomatic heart failure and about one percent in the control arm. That’s less than the four percent incidence attributable to trastuzumab that we needed to see to continue the study (3.1). We also found that approximately 25 percent of patients weren’t completing the full year of trastuzumab due to asymptomatic drops in LVEF that mandated discontinuation.
Approximately four percent of patients who received trastuzumab are still taking medications to manage heart failure, but they’re not symptomatic. We tracked the patients carefully, following up every six months to determine whether their symptoms persisted, the status of their LVEF and whether they were still on medication. Only one of the patients who developed symptoms remains symptomatic.
Approximately nine and a half percent of patients on the trastuzumab arm and four and a half on the control arm had ejection fractions lower than 50 at 18 months, so we’ve learned that sequential AC  paclitaxel has some impact on long-term cardiac function, which is why the control arm is so critical in this trial. paclitaxel has some impact on long-term cardiac function, which is why the control arm is so critical in this trial.
Reversibility of declines in LVEF
A substantial improvement in ejection fractions occurs across the board, with virtually all patients then moving back toward baseline. A slight downward shift of the distribution occurs in a small number of patients with LVEFs in the 40 to 50 percent range, and a couple of patients in the upper 30 percent range. Many of the patients with LVEFs less than 40 percent had recent events and have not yet had time to recover; however, the ejection fractions do recover substantially.
Potential implications for nonprotocol treatment
We collected information on known cardiac risk factors for all patients enrolled in the study. Patients had to have a normal EKG and no history of cardiac events. On the cardiac safety study, only 15 percent of patients were older than age 60.
This is a select group of healthy patients with normal cardiac function, which will be one of the many dilemmas when we start seeing patients who would not have met the eligibility criteria of the study, but whom we know would benefit from trastuzumab. It will be challenging to figure out how to extrapolate the data to patients who might have some pre-existing cardiac dysfunction.
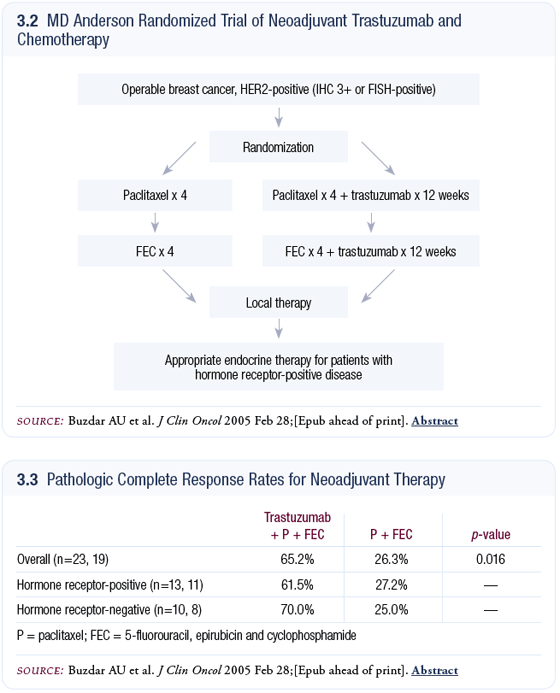
MD Anderson clinical trial of neoadjuvant trastuzumab
The pCR rate of 65 percent is phenomenal (Buzdar 2004; [3.3]). Interestingly, the rationale for doing the study was that they disagreed with the decision to not continue studying trastuzumab combined with anthracyclines.
They adapted their backbone regimen of paclitaxel followed by FAC and utilized FEC 75. They also made the decision to truncate trastuzumab to 24 weeks (3.2). In a number of other neoadjuvant trastuzumab studies — primarily with vinorelbine but also with carboplatin/paclitaxel — the typical pCR was 20 percent to 30 percent. Steve Limentani pushed it up to 35 percent with vinorelbine/docetaxel, but clearly the MD Anderson regimen dramatically outperforms those combinations.
It intrigues me that they took two sequential regimens that presumably interact well with trastuzumab and administered them sequentially. Their regimen was much longer in duration than the other regimens. If you evaluate the nontrastuzumab data, you see the same trend of higher pCR rates associated with longer duration of therapy.
I can’t help but wonder whether their results are due to the epirubicin/ trastuzumab combination or the two sequential approaches? That’s an extremely important question. I would bet the combination is important for some patients — perhaps those who co-overexpress topoisomerase II and HER2. But, is it good for all patients? The MD Anderson study probably generates more questions than it answers.
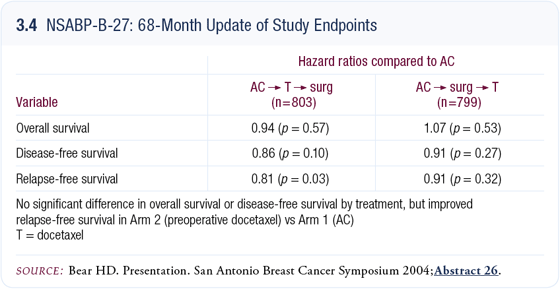
NSABP trial B-27: Neoadjuvant AC/docetaxel
This was a three-arm study in which all patients received neoadjuvant therapy. The control group was AC for four cycles followed by surgery. The second group was AC followed by docetaxel followed by surgery. The third group had surgery between the AC and the docetaxel (Bear 2003, 2004).
We previously reported a doubling of pCR rates in the second group of patients who received docetaxel before surgery. Earlier this year, a sufficient number of events had occurred on study to proceed with the final definitive survival analysis. Surprisingly, overall survival was no different among the three arms. In terms of disease-free survival, slightly fewer events occurred among the patients receiving docetaxel, but it was not statistically significant — and this was mature data with approximately 700 events (3.4). In evaluating B-27, according to our planned analysis, it was a negative trial.
The pCR has not yet been shown to be a surrogate for long-term outcome. I believe pCR remains a valid investigational tool for trying to sort out improved therapies, but we still have to investigate these therapies in large adjuvant trials.
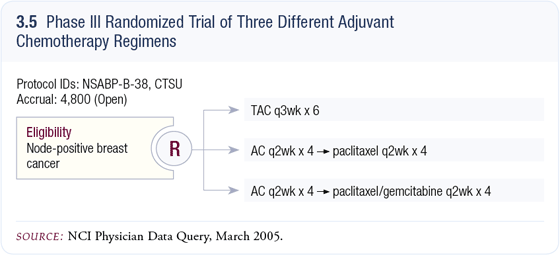
NSABP-B-38: Phase III adjuvant trial comparing three chemotherapy regimens in women with node-positive breast cancer
Two key adjuvant trials have been BCIRG-001, evaluating TAC versus FAC (Martin 2003), and the CALGB dose-dense trial 9741 of AC paclitaxel (Citron 2003). Currently, our view is that TAC appears to be the optimal way to administer an anthracycline/docetaxel regimen and dose-dense AC paclitaxel (Citron 2003). Currently, our view is that TAC appears to be the optimal way to administer an anthracycline/docetaxel regimen and dose-dense AC  paclitaxel is the optimal way to administer those agents. paclitaxel is the optimal way to administer those agents.
Which is better? It’s impossible to answer that question without performing a clinical trial, which is why we developed trial NSABP-B-38. It’s a pragmatic design in which we regard TAC as our control arm (3.5).
A clear advantage of dose-dense therapy is that it is so well tolerated, and it clearly affords the opportunity to add a fourth drug to the paclitaxel. TAC is a maximally tolerated regimen. You really can’t push it much more, so we sought a candidate drug to combine with paclitaxel. The study of paclitaxel/gemcitabine versus paclitaxel in metastatic breast cancer reported at ASCO demonstrated an improved response rate, time to progression and overall survival (Albain 2004).
Obviously, those results peaked our interest, but a number of investigators have been evaluating dose-dense paclitaxel with gemcitabine. Dr Colomer from Spain performed a Phase II study in patients with untreated metastatic breast cancer and demonstrated an overall response rate of 71 percent, with a 26 percent complete response rate and a remarkable safety profile (Colomer 2004). He used 2,500 mg/m2 of gemcitabine every two weeks combined with 150 mg/m2 of paclitaxel, and it was well tolerated. Those two data sets suggested it would be ideal to bring into the adjuvant setting because it could be added to Dr Norton’s dose-dense regimen.
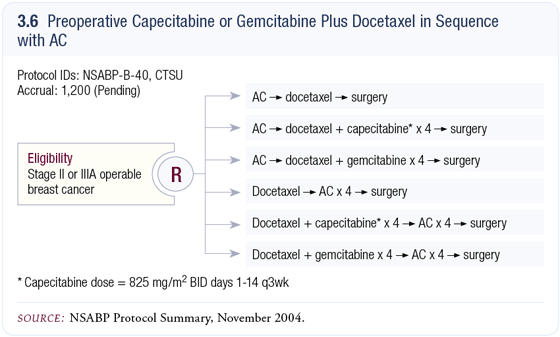
NSABP-B-40 neoadjuvant trial
NSABP-B-40 is the replacement trial for NSABP-B-27. We will continue using sequential AC followed by docetaxel as our control, with a second arm utilizing capecitabine/docetaxel following AC and a third arm with gemcitabine/docetaxel also following AC (3.6). The data with capecitabine/docetaxel in the metastatic setting is compelling because survival advantages in metastatic disease usually translate into benefit in the adjuvant setting.
The notion that docetaxel is better than paclitaxel has changed with the results of B-27, but we believe continued investigation is warranted. We would like to continue to work with docetaxel combined with capecitabine in the neoadjuvant setting.
Our problem is we have so many drugs that are active, but we need to figure out how to identify predictive factors. Docetaxel is an extremely important drug for some patients, but others derive no benefit. Our neoadjuvant program is attempting to identify those predictive factors so we can utilize the right drug in the right patient.
Select publications
 |
Dr Geyer is the Director of Medical Affairs of the National Surgical Adjuvant Breast and Bowel Project and Director of Breast Medical Oncology at Allegheny General Hospital in Pittsburgh, Pennsylvania. |
|

