You are here: Home: BCU 3 | 2005: Power Point Journal Club
| |
|
|
| |

This PowerPoint Journal reviews recently published clinical research articles and presentations. In this issue, we review papers by Howard Burris, MD and colleagues evaluating a Phase II study of trastuzumab followed by weekly paclitaxel and carboplatin as first-line therapy for patients with metastatic breast cancer and a status report by Eric Winer, MD et al on the ASCO Technology Assessment on the use of aromatase inhibitors as adjuvant therapy for women with hormone receptor-positive breast cancer.
These PowerPoint Journal Club slides are provided in different formats in this monograph and on the enclosed enhanced CD. The slide presentation on the CD was designed for optimal viewing on a large screen in a dark room (below, right) and represents top-line data and information from the figures in this book. The PowerPoint file and PDF file of this monograph can be accessed at www.BreastCancerUpdate.com.
| 6.1 |
|
| |
|

SLIDE 6.1 Preclinical studies have demonstrated that platinum and taxanes are additive or synergistic with trastuzumab and increase the response rate over that which was reported with either agent alone. The current Phase II trial evaluates this triplet regimen as first-line therapy.
|
| |
|
|
| 6.2 |
|
| |
|
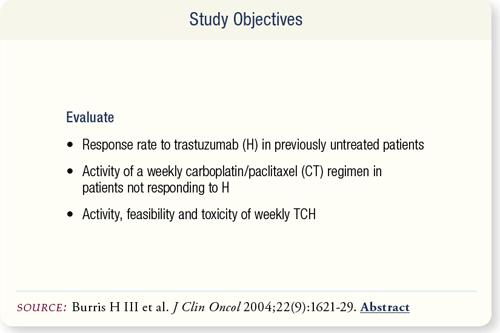
SLIDE 6.2 This study sought to determine the feasibility of single- agent trastuzumab (H) as first-line therapy in patients with HER-2 positive metastatic breast cancer, the activity of weekly carboplatin/paclitaxel in patients unresponsive to H, and the activity and toxicity of trastuzumab/carboplatin/paclitaxel.
|
| |
|
|
| 6.3 |
|
| |
|
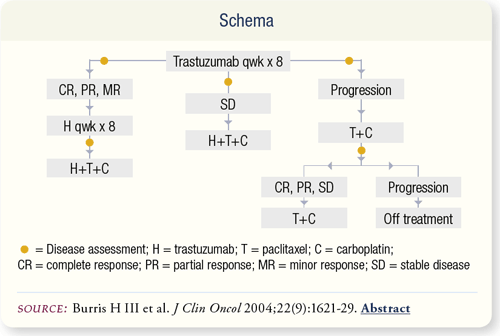
SLIDE 6.3 Trastuzumab (H) was administered weekly for the first eight weeks. Responders (CR, PR or MR) continued H for another eight weeks, after which weekly paclitaxel/carboplatin (TC) was added. Patients who had stable disease received eight-week cycles of six-weekly TCH.
|
| |
|
|
| 6.4 |
|
| |
|
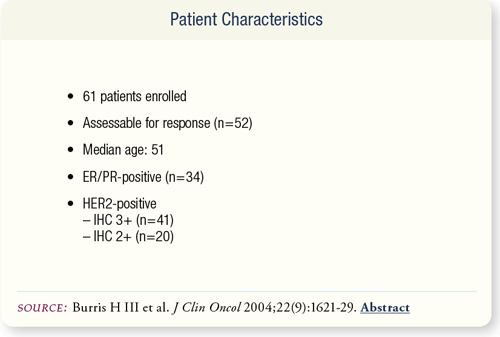
SLIDE 6.4 Sixty-one patients were enrolled in the study, and all were assessable for survival and safety. Six patients did not meet criteria for measurable disease and three patients prematurely discontinued the study, resulting in 52 patients assessable for disease response.
|
| |
|
|
| 6.5 |
|
| |
|
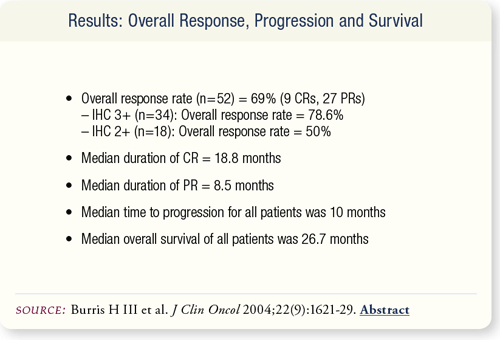
SLIDE 6.5 The overall response rate including all treatments was 69 percent, with a median duration of complete response of 18.8 months and a median duration of partial response of 8.5 months.
|
| |
|
|
| 6.6 |
|
| |
|
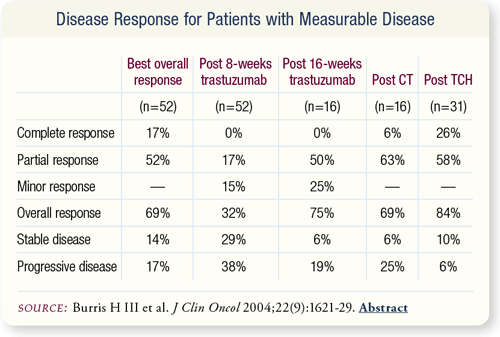
SLIDE 6.6 Approximately 32 percent of patients had a minor/partial response to trastuzumab (H) and received eight more weeks of H, and 29 percent of patients had stable disease and received TCH, with an overall response rate of 84 percent. Patients treated with CT after progression on initial H had an overall response rate of 69 percent.
|
| |
|
|
| 6.7 |
|
| |
|
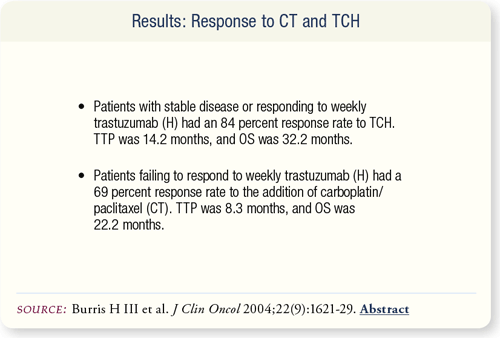
SLIDE 6.7 Treatment of patients with TCH resulted in a response rate of 84 percent with median time to progression (TTP) and overall survival (OS) of 14.2 and 32.2 months, respectively. Sixteen of the 20 nonresponders to weekly H were treated with CT, with resulting response rates of 69 percent.
|
| |
|
|
| 6.8 |
|
| |
|
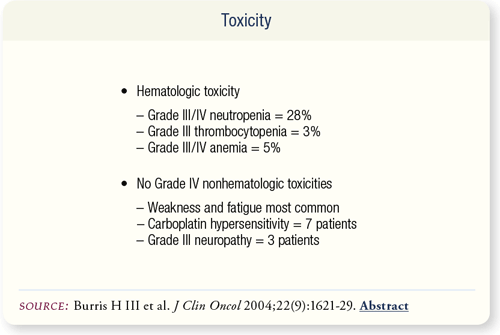
SLIDE 6.8 Chemotherapy was well tolerated. Nineteen patients had doses held primarily due to myelosuppression. Anemia, neurotoxicity, fatigue and edema were the other causes of delayed doses. No febrile neutropenia was reported.
|
| |
|
|
| 6.9 |
|
| |
|
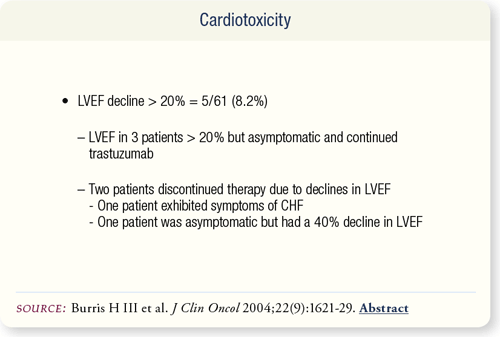
SLIDE 6.9 Five of 61 patients experienced a decline in ejection fraction. One patient with a 40 percent decline continued on carboplatin and paclitaxel without trastuzumab. Her ejection fraction subsequently recovered to 50 percent. The overall cardiotoxicity rate was eight percent.
|
| |
|
|
| 6.10 |
|
| |
|
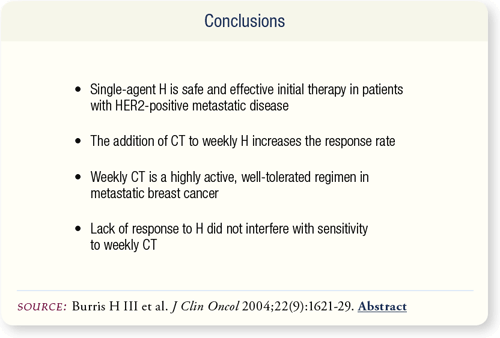
SLIDE 6.10 This study confirmed the single-agent activity of trastuzumab and the benefit of carboplatin/paclitaxel with or without trastuzumab in patients with HER-positive metastatic disease.
|
| |
|
|
Select publications
| 7.1 |
|
| |
|

SLIDE 7.1 The ASCO technology assessment is conducted by a multidisciplinary panel of experts who review and synthesize the latest available data in order to make recommendations on therapeutic approaches in clinical practice.
|
| |
|
|
| 7.2 |
|
| |
|
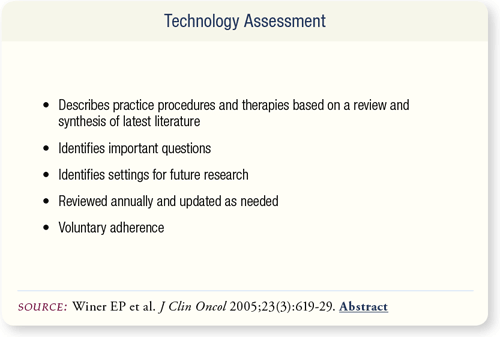
SLIDE 7.2 The technology assessment is a process that follows defined ASCO policies and procedures for determining whether a procedure is appropriate for broad-based conventional use in clinical practice. It is reviewed and updated annually. Adherence to the guidelines is voluntary.
|
| |
|
|
| 7.3 |
|
| |
|
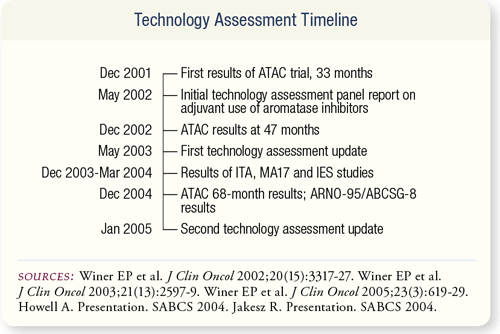
SLIDE 7.3 The first results of the ATAC trial presented the oncology community with a new approach to the adjuvant therapy of postmenopausal women with hormone-responsive breast cancer. The ASCO technology assessment was formed soon after in order to review the data and provide recommendations on the adjuvant use of aromatase inhibitors.
|
| |
|
|
| 7.4 |
|
| |
|
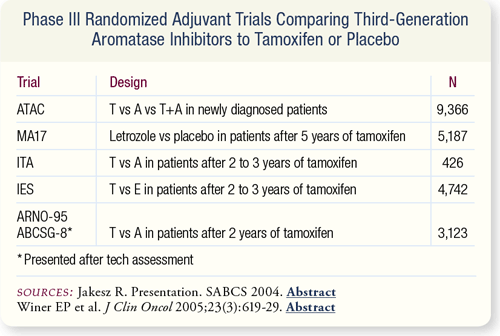
SLIDE 7.4 Since the publication of the last panel update in 2003, the results of five randomized trials comparing third-generation aromatase inhibitors (AI) to tamoxifen were presented. As in the ATAC trial, they demonstrated improved benefit of AIs over tamoxifen in rates of disease recurrence.
|
| |
|
|
| 7.5 |
|
| |
|
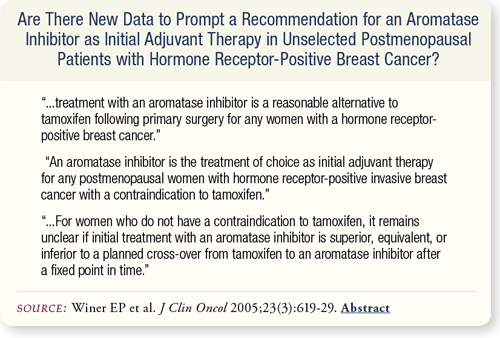
SLIDE 7.5 Based on the results of multiple large randomized trials, the panel recommends the inclusion of an aromatase inhibitor as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer.
|
| |
|
|
| 7.6 |
|
| |
|
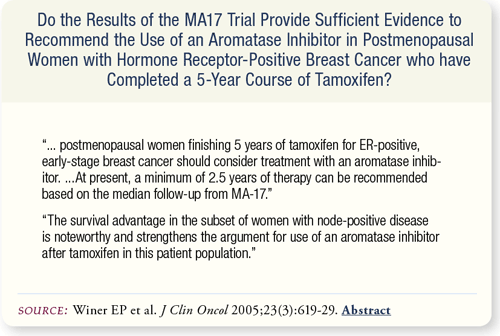
SLIDE 7.6 Based on the 2.5 years median follow-up of the MA17 study, the panel recommends that postmenopausal women with ER-positive breast cancer finishing five years of adjuvant tamoxifen should consider treatment with an aromatase inhibitor for a minimum of 2.5 years.
|
| |
|
|
| 7.7 |
|
| |
|
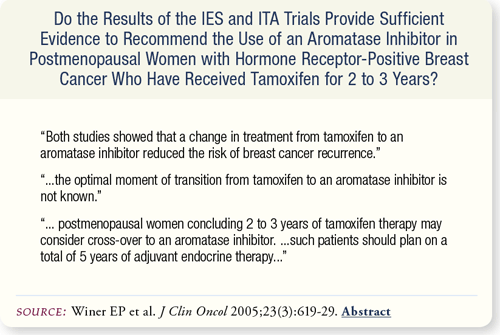
SLIDE 7.7 Both the IES and ITA trials showed a reduction in breast cancer recurrence risk following a change in treatment from tamoxifen to an aromatase inhibitor. However, the optimal time of treatment transition is unknown.
|
| |
|
|
| 7.8 |
|
| |
|

SLIDE 7.8 While there are studies underway, there is no present data to support the continuation of aromatase inhibitors beyond five years. The panel does not recommend treatment with an aromatase inhibitor for longer than five years outside of a clinical trial.
|
| |
|
|
| 7.9 |
|
| |
|
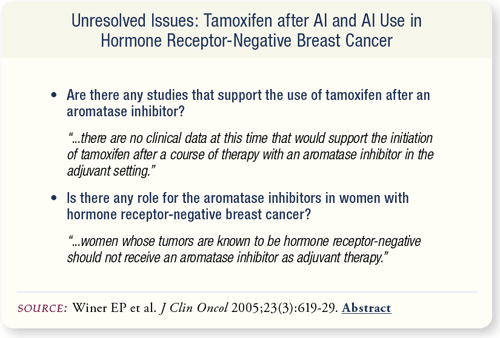
SLIDE 7.9 No existing data support the use of tamoxifen after an AI, and women completing initial adjuvant therapy with an AI should not be crossed over to tamoxifen outside of a clinical trial. However, if a woman develops toxicity on initial treatment with an AI, it is not unreasonable to switch to tamoxifen.
|
| |
|
|
| 7.10 |
|
| |
|
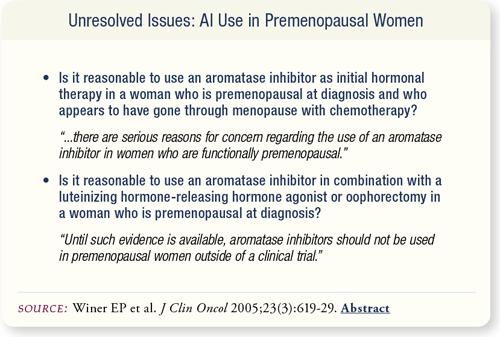
SLIDE 7.10 Because of the lack of evidence for adequate estrogen suppression and the potential for increased gonadotropin release stimulating the ovaries, aromatase inhibitors should not be used either as monotherapy or in combination with ovarian function suppression in premenopausal women outside of a clinical trial.
|
| |
|
|
| 7.11 |
|
| |
|
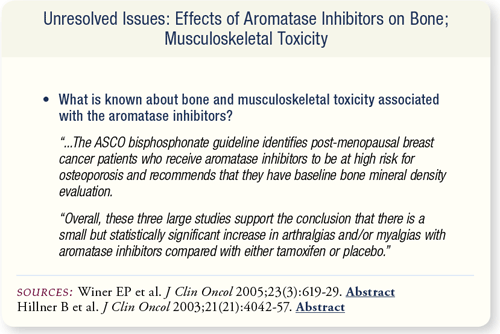
SLIDE 7.11 In all the studies reviewed by the technology assessment panel, the use of AIs was associated with increases in fractures, arthralgias and/or myalgias. The ASCO bisphosphonate guidelines recommend that breast cancer patients with a high risk of osteoporosis have bone mineral density evaluated.
|
| |
|
|
| 7.12 |
|
| |
|
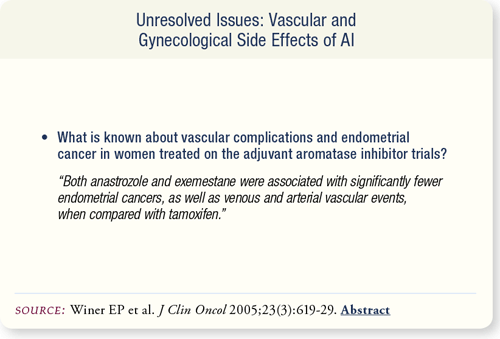
SLIDE 7.12 There were significantly fewer occurrences of endometrial cancers, pulmonary emboli and stroke in women treated with anastrozole or exemestane when compared to women treated with adjuvant tamoxifen.
|
| |
|
|
| 7.13 |
|
| |
|
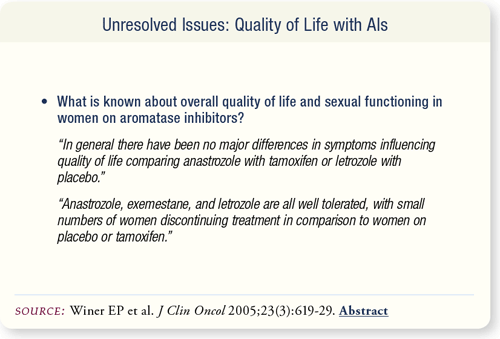
SLIDE 7.13 Comparison of patient-perceived symptoms with AIs is difficult due to a lack of standard criteria for data collection and the differences in clinical situations. In general, there do not seem to be major differences in the quality of life when comparing anastrozole with tamoxifen or letrozole with placebo.
|
| |
|
|
| 7.14 |
|
| |
|

SLIDE 7.14 The differences in absolute benefit that a woman may expect are important considerations in the decision-making process and the technology assessment panel recommends that each patient’s individual circumstance be considered when making recommendations.
|
| |
|
|
| 7.15 |
|
| |
|
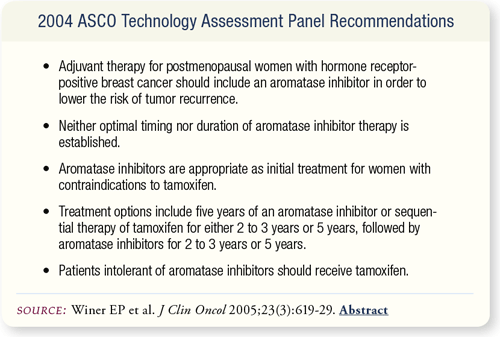
SLIDE 7.15 These points summarize the recommendations of the 2004 ASCO technology assessment panel.
|
| |
|
|
| 7.16 |
|
| |
|
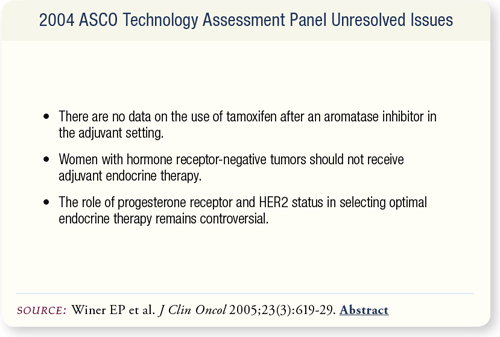
SLIDE 7.16 A number of important questions and issues remain unresolved at this time mainly because of a lack of data.
|
| |
|
|
| 7.17 |
|
| |
|
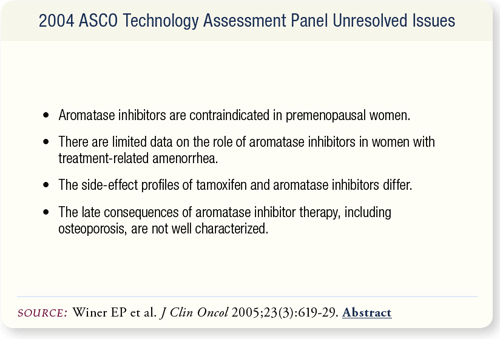
SLIDE 7.17 Many of these unresolved issues will be addressed by ongoing studies and additional follow-up. As more data become available, their impact will be reflected in future technology assessment updates.
|
| |
|
|
Select publications
|
|
| |
|
|
| |
|
|
| |
|
|
|
|

|
|
|

