After 30 years of studying cancer biology and knowing all the different pathways and growth factor signaling, we’re at a very exciting point where we may actually use combination targeted therapies that will be different for different patients. We have to move away from giving the same treatment to everybody. It’s all a mindset. We have to give the right agent to a small subset of patients.
Also, clinical trialists have to accept the idea of assessing the activity of these agents in patients with earlier stage disease — perhaps Stage IIIB, or first-line metastatic — as soon as we have demonstrated safety in clinical trials. If you use targeted agents in heavily pretreated patients with metastatic disease, the activity will be very low.
This is the development process for targeted therapies. It’s a new era and it takes time because people are still stuck doing large metastatic studies looking for a signal, and then slowly moving forward. That takes a long time for women, if you think about it.
— Jenny C Chang, MD
We have a tendency to divide all trial outcomes into either positive or negative when, in fact, most of what we generate is noninformative.
— I Craig Henderson, MD |
Every now and again I get cranky about the glacier-like pace of cancer research. Recently, I was whining about this to a well-known “translational scientist,” and his somewhat defensive response focused on the measly two to three percent of patients participating in clinical trials and the great need to increase trial accrual. Sure, blame it on the patients!
Clinical research can be viewed as a market-driven business, and the supply-and-demand concept should have the same validity as in selling fried chicken. I would wager that better financial compensation to physicians and maybe even to patients would solve a lot of the problem lickety split.
I guess it’s too much to ask for something simple like a fee increase, so we will need to continue relying on the unselfishness and altruism of patients (and their physicians) who are willing to participate in clinical research.
If I had cancer (God forbid, as they say), participation in a clinical trial would be inviting for at least two reasons:
- It would provide additional assurance that my therapy would be within the standards of excellence. I don’t fully trust anyone anymore, including myself, and the more eyes on my chart the better.
- Maybe we will learn something useful that might benefit me and my fellow patients.
With regard to motivation #2, it would be a lot more exciting to be part of a Jenny Chang-like neoadjuvant trial in which my tumor would be carefully studied and correlated with my clinical course, than to enter another 3,000-patient adjuvant extravaganza in which I might avoid the key event that results in a statistically significant p-value. (Although that would be fine also.)
I also like the idea of being enrolled in a Phase II trial of a novel molecularly targeted agent or combination like the bevacizumab-erlotinib study described by Maura Dickler in this issue of Breast Cancer Update. Neither of those agents is likely to make me ill, and who knows what might happen?
Surprises can and do occur in Phase I and II studies, and Craig Henderson speaks about this phenomenon when he recounts the pivotal Phase II trial of trastuzumab in the early 1990s. “The most important and exciting study I’ve done in my career,” says Craig, who describes the work-related euphoria he felt when administering this highly targeted, relatively nontoxic, scientifically compelling therapy and seeing tumors shrink.
Craig describes an impressive response to trastuzumab in a woman with massive ascites and liver metastases, and he notes that you don’t have to be a rocket scientist to know that this type of observation — even if only in a handful of patients — is a signal we can’t ignore.
Drs Chang and Dickler give me hope that other new advances are around the corner...or at least in the neighborhood.
In the neoadjuvant trial of women with HER2-positive tumors that Dr Chang first reported at the 2003 San Antonio Breast Cancer Symposium, 25 percent of patients experienced a partial tumor response after just three weekly doses of trastuzumab. “It was stunning,” she said. Most of the patients were indigent women presenting with locally advanced breast cancer.
Given that the patients in Dr Chang’s study had such large tumors, a 50 percent decrease in measurable diameter in any tumor in three weeks is remarkable for a nontoxic molecularly targeted therapy. It is ironic that these patients, suffering from poverty — both personally and oncologically — have been part of the vanguard of a new area of clinical research.
It is also sobering to consider that the basic trial concept of sequential biopsies while administering trastuzumab had not previously been implemented, although tens of thousands of patients have now received this landmark agent. Dr Chang argues persuasively that in the future, promising targeted therapies must be tested much sooner in the neoadjuvant setting, and this strategy makes sense.
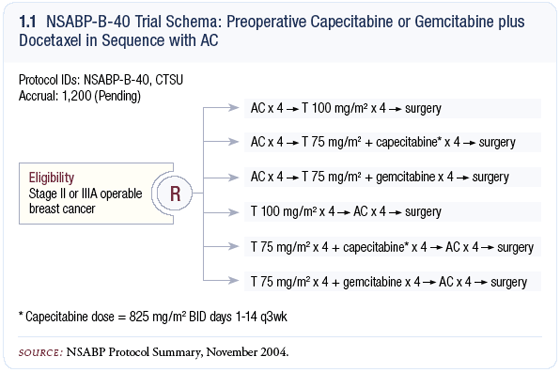
So let’s do it! NSABP-B-40 — a neoadjuvant trial with major emphasis on tissue correlation — is about to be launched (1.1). Every surgeon and oncologist in this country can enter patients through the CTSU www.CTSU.org. Let’s commit to enroll patients with newfound zeal. Maybe we should decrease the frequency of television ads for erectile dysfunction medications by 10 percent and invest those dollars in promoting B-40. Call it a societal tax. Whatever, let’s just get the study done now.
I tried to convince Richard Peto on this program that the concept of clinical research that results in modest advances in frequent tumors with high mortality rates is getting boring. He, however, rightfully points to the projected halving of breast cancer mortality from 1990 to 2010 as supporting the stepwise approach to progress. Okay, I can’t argue with that, so let’s do much more of both megarandomized Phase III trials and clever, strategic, tissue-correlated Phase I and II studies...and let’s do that a lot sooner than later.
— Neil Love, MD
NLove@ResearchToPractice.net
|

