|
Just before boarding a peanut-and-pretzels-only flight to Atlanta for the Society of Surgical Oncology meeting, I received an email from our scientific director, Rick Kaderman. Attached were two interesting JCO articles* that had just become available online. The first was the formal publication of Aman Buzdar’s neoadjuvant trastuzumab study, which was initially presented at the 2004 ASCO meeting. The second was the accompanying editorial by Harold Burstein and Eric Winer.
That evening, while my wife Adriana and I were dining at the somewhat unappetizing Atlanta Hyatt lobby buffet, Aman — who was to join me the next morning on a tumor panel discussion at the ungodly surgical hour of 6:00 AM — dropped by our table. He had just arrived back from Japan where he was doing a visiting professorship, during which he spent some time in Hiroshima. All he could talk about was the emotional enormity of being in the place where so many people died instantly. While I listened intently to his travel-related stories, I was also curious about the JCO paper. “The editors contacted me right after ASCO,” he said. “They wanted to see it published quickly.”
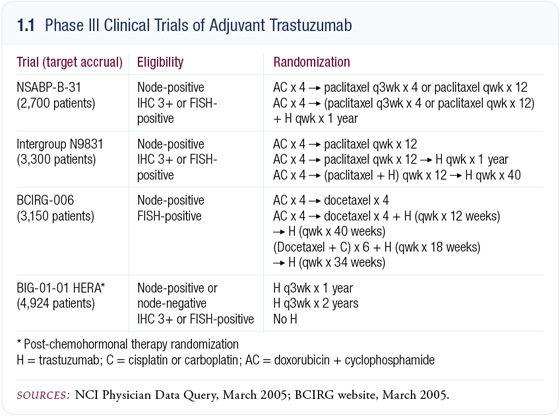
No wonder. The importance of Aman’s study was eloquently discussed by Hal and Eric in an extended editorial, which noted that the day is soon coming when HER2-positive breast cancer will truly be considered a separate disease, and the remaining HER2-negative patient subset will look a lot different. Very specifically, Aman’s study sets the stage for the most anticipated group of trials in breast cancer clinical research in the last decade: the four large randomized studies evaluating adjuvant trastuzumab (1.1).
The next issue of our series includes an extraordinary interview with Edith Perez, the principal investigator of one of these landmark studies, NCCTG-9831. After major prodding on my behalf (which made me feel like a prosecuting attorney), Edith spilled some major beans: The NCI and FDA have just agreed to allow the data from the two common randomization arms of N9831 and NSABP-B-31 to be combined into one analysis.
According to Edith, this data set will be analyzed in April and has enough events to provide an initial evaluation of the risks and benefits of adjuvant trastuzumab. With more arm twisting (sorry, Edith!), she told me that the results could become publicly available as early as this summer, although it could also be much longer before we hear anything due to very stringent statistical boundaries for revealing the data at this point. The other two major trastuzumab trials (HERA and BCIRG- 006) might not have results for a year or two.
The eternal optimist in me (and all oncologists) says that things won’t be the same in breast cancer after the unprecedented NCCTG-NSABP analysis. This situation reminds me of the months leading up to the first presentation of the ATAC data in December 2001. As with the discussion with Edith, I received an early “heads up” about ATAC during an interview with Mike Baum in February 2001 at the Miami Breast Cancer Conference. At that time, no one had a clue when the initial data would be analyzed, but Mike revealed that the trialists had just determined that enough events had transpired to perform a data analysis that November and present the findings the following month in San Antonio.
From that point on, one of my standard questions during any interview for this series was, “What do you think the ATAC trial will show?” All but one person, who now lives in infamy (sorry, Bob!), predicted without much hesitation that anastrozole would be superior to tamoxifen, and that indeed, is exactly what occurred. Most of these investigators also commented that bone would likely be an issue because bone density monitoring and the use of bisphosphonates were not included in the ATAC protocol.
Of course, many other times in the history of breast cancer clinical research our hopes and expectations have been crushed by trial results — witness the rise and fall of stem cell transplantation — but ATAC and the other aromatase inhibitor trials have provided renewed confidence that advances in metastatic disease will translate to the adjuvant setting.
As the little ball on the adjuvant trastuzumab roulette wheel is slowly coming to a halt, and we hold our collective breaths in anticipation, I have adopted a new favorite interview question, “What do you think the adjuvant trastuzumab trials will show?” So far, the results have been unanimously optimistic, and I am also fully on the adjuvant H bandwagon.
Nothing in oncology will make sense anymore if these trials don’t show at least a significant reduction in the short-term recurrence rate with trastuzumab, particularly in view of studies like Aman’s neoadjuvant trial, which clearly demonstrates a major bump in tumor control by adding this landmark targeted agent. Even with a three to four percent rate of cardiac toxicity with trastuzumab, a relative reduction in relapse rate of even 20 to 30 percent will result in a positive benefit-to-risk ratio for patients with HER2-positive, node-positive tumors, particularly those lacking estrogen and progesterone receptors.
The answers will start appearing soon, and if things transpire as expected, Hal and Eric’s concept of HER2-positive breast cancer as a separate disease entity will be fully on the table. I can’t imagine that it won’t be quickly embraced, but it is also fascinating to consider how the residual non-HER2 tumors will be reconceptualized and how all of this ties in with new classification systems such as those related to the Genomic Health Oncotype DX™ assay and to the work of Charles Perou. All four speakers in this issue of Breast Cancer Update comment on this issue, which is perhaps the most discussed topic in breast cancer research today.
With all this being said, it is clear that the HER2 overture is over and the symphony is about to begin. Patients and physicians will be on the edge of their seats and I hope and pray they will not be disappointed.
— Neil Love, MD
NLove@ResearchToPractice.net
Select publications
Carey LA et al. The triple negative paradox: Primary tumor chemosensitivity of the basal-like breast cancer (BBC) phenotype. San Antonio Breast Cancer Symposium 2004;Abstract 1023.
Paik S et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351(27):2817-26. Abstract
Perou CM et al. Molecular portraits of human breast tumors. Nature 2000;406(6796):747-52. Abstract
Rouzier R et al. Basal and luminal types of breast cancer defined by gene expression patterns respond differently to neoadjuvant chemotherapy. San Antonio Breast Cancer Symposium 2004;Abstract 201.
* Buzdar AU et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: Results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 2005;23(16);[Epub ahead of print]. Abstract
Burstein HJ, Winer EP. HER2 or not HER2: That is the question. J Clin Oncol 2005;23(16); [Epub ahead of print]. No abstract available
|

