You are here: Home: BCU 2| 2005: Power Point Journal Club
| |
|
|
| |

The PowerPoint Journal Club will review important, recently published articles and meeting presentations. In the current issue, we a review a paper by Edwin Fisher and colleagues evaluating scoring methods for estrogen and progesterone receptors and an article by Soonmyung Paik and colleagues about the Oncotype DX™ multigene assay. We also review data presented by Raimund Jakesz at the 2004 San Antonio Breast Cancer Symposium regarding ABCSG trial 8 and ARNO trial 95 of switching postmenopausal women to anastrozole after two years of adjuvant tamoxifen.
PowerPoint presentations are provided in two different formats: in print and on CD (see the thumbnails below). The CD versions of the PowerPoint presentations were designed for optimal viewing on a large screen in a dark room (below, right). This design can be difficult to read in print, and consequently the print versions have been designed to facilitate ease of reading.
| 7.1 |
|
| |
|

SLIDE 7.1 The ligand-binding, dextran-coated charcoal (DCC) technique, which quantitatively estimates estrogen receptors (ER) and progesterone receptors (PR), has been used to derive most of the data about prognosis and tumor response after tamoxifen. For numerous reasons, immunohistochemistry (IHC) has largely replaced DCC for the detection and quantification of ER and PR.
|
| |
|
|
| 7.2 |
|
| |
|

SLIDE 7.2 A number of methods are used for scoring IHC results as either positive or negative. In this article, Fisher et al attempt to resolve issues about the lack of consensus of an optimal IHC method. The objective of this study was to correlate different IHC and DCC estimates for ER and PR with overall, disease-free and recurrence-free survival in patients enrolled in NSABP-B-09.
|
| |
|
|
| 7.3 |
|
| |
|
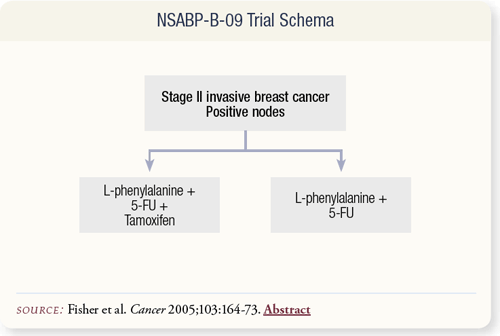
SLIDE 7.3 NSABP-B-09 randomly assigned 1,891 women with node-positive, Stage II breast cancer to two years of adjuvant L-phenylalanine/5-FU with or without tamoxifen. ER status was assessed by DCC, and values of >10 fmol/mg were considered positive. This analysis included 402 patients with adequate pathologic material in both arms of the study.
|
| |
|
|
| 7.4 |
|
| |
|
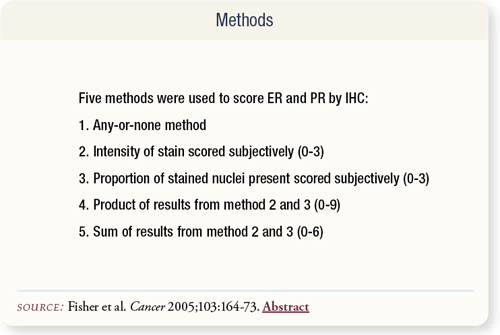
SLIDE 7.4 Five IHC scoring methods were used on this test set: any-or-none (any proportion of any positive degree of intensity considered positive), intensity of stain, proportion of stained nuclei present, product of second and third methods and sum of second and third methods.
|
| |
|
|
| 7.5 |
|
| |
|
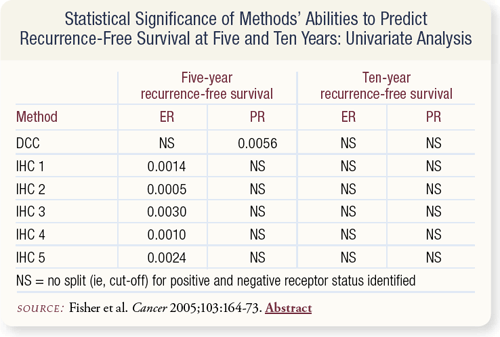
SLIDE 7.5 ER-positivity measured only by IHC methods utilizing any-or-none and intensity of staining related significantly to a favorable five-year disease-free survival (DFS). PR-positivity measured only by DCC was significantly related to five-year and 10-year DFS.
|
| |
|
|
| 7.6 |
|
| |
|
SLIDE 7.6 ER-positivity measured by any IHC methods related significantly to a favorable five-year recurrence-free survival (RFS). ER-positivity was not significantly related to a favorable 10-year RFS. PR-positivity measured only by DCC was significantly related to five-year RFS and was not significantly related to 10-year RFS.
|
| |
|
|
| 7.7 |
|
| |
|
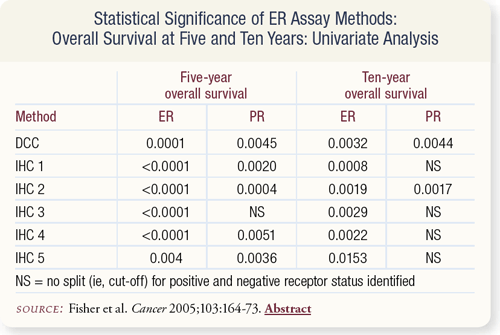
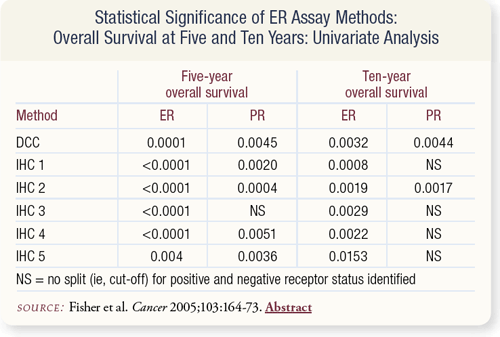
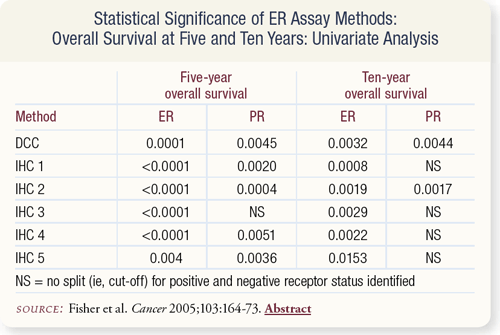
SLIDE 7.7 ER-positivity measured by DCC and five IHC methods related significantly to a favorable five- and 10-year overall survival (OS). For 10-year OS, PR-positivity measured only by DCC and the IHC method utilizing intensity of staining was significantly related to OS.
|
| |
|
|
| 7.8 |
|
| |
|
SLIDE 7.8 Interobserver agreement for IHC estimates and concordance between DCC and the IHC methods were good. On univariate analysis, ER-positivity related to favorable five- and 10-year overall survival. On multivariate analysis, ER-positivity related to favorable five- and 10-year overall survival in patients with an unfavorable lymph-node status.
|
| |
|
|
| 7.9 |
|
| |
|
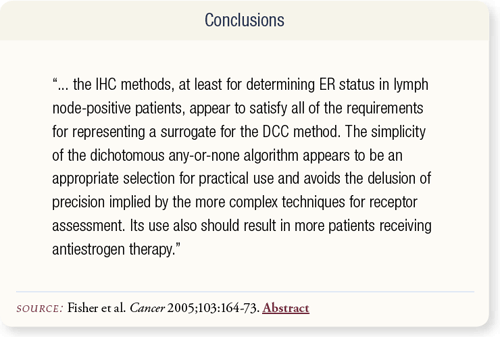
SLIDE 7.9 Fisher et al conclude that the any-or-none IHC method for assessing ER status seems to be a good test to use in clinical practice.
|
| |
|
|
| |
|
Select publications |
| |
|
|
| 8.1 |
|
| |
|

SLIDE 8.1 The distant recurrence rate in women with ER-positive, node-negative breast cancer has not been well defined.
|
| |
|
|
| 8.2 |
|
| |
|
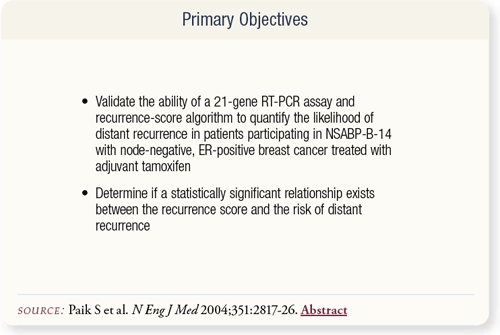
SLIDE 8.2 Paik et al evaluated a 21-gene reverse-transcriptasepolymerase- chain-reaction (RT-PCR) assay. The objective was to determine if the recurrence-score (RS) algorithm predicted the distant recurrence rate in patients who participated in NSABP-B-14 receiving tamoxifen
|
| |
|
|
| 8.3 |
|
| |
|
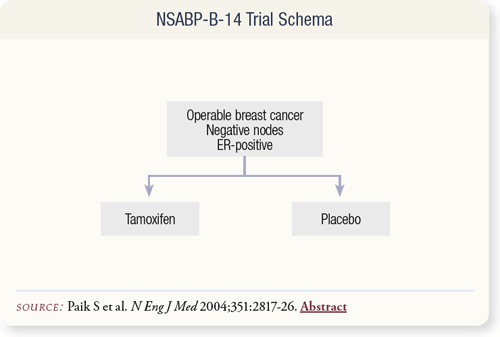
SLIDE 8.3 NSABP-B-14 enrolled 2,892 women with operable, ER-positive, node-negative, primary breast cancer who were randomly assigned to adjuvant therapy with tamoxifen or placebo. An additional 1,235 women were enrolled and received adjuvant tamoxifen. This analysis included paraffin blocks from 668 of the 2,617 women treated with adjuvant tamoxifen.
|
| |
|
|
| 8.4 |
|
| |
|
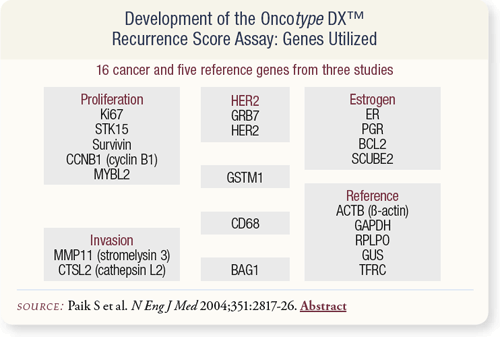
SLIDE 8.4 These 21 genes — selected from three preliminary studies with 447 patients and 250 candidate genes — were used to determine the recurrence score.
|
| |
|
|
| 8.5 |
|
| |
|
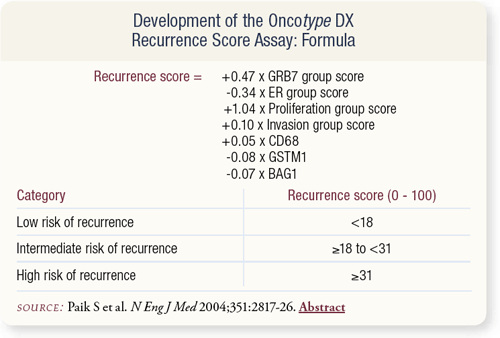
SLIDE 8.5 The algorithm in this slide determined the recurrence score. The range of recurrence scores (RS) was 0 to 100. Patients were classified into the following categories: low risk (RS<18), intermediate risk (18≤RS<31) and high risk (RS≥31).
|
| |
|
|
| 8.6 |
|
| |
|
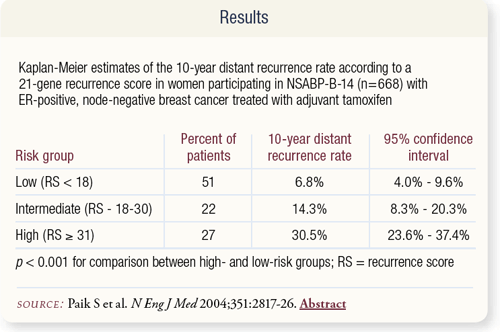
SLIDE 8.6 The Oncotype DX assay was used to measure gene expression for 668 patients enrolled on NSABP-B-14. Fiftyone percent, 22 percent and 27 percent of the patients were categorized as low, intermediate and high risk, respectively. Kaplan-Meier estimates for 10-year distant recurrence rates were significantly lower for the low-risk than the high-risk group.
|
| |
|
|
| 8.7 |
|
| |
|
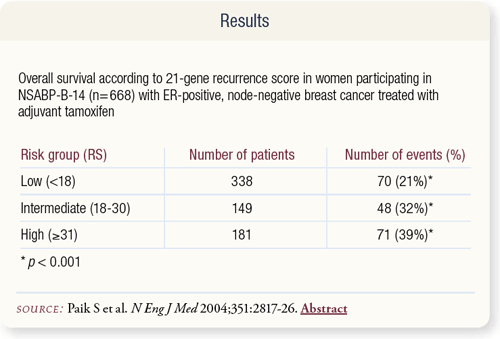
SLIDE 8.7 The RS was also significantly correlated with overall survival (p < 0.001). The data presented in this table are found in the appendix to the article by Paik et al.
|
| |
|
|
| 8.8 |
|
| |
|
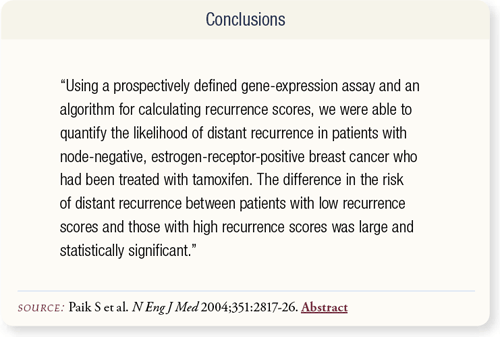
SLIDE 8.8 The calculated RS predicted the likelihood of distant recurrence and overall survival in patients with ER-positive, node-negative breast cancer treated with adjuvant tamoxifen. At the 2005 San Antonio Breast Cancer Symposium, Paik et al presented data demonstrating the RS is also able to predict the benefit with adjuvant chemotherapy in this patient subset.
|
| |
|
|
| |
|
Select publications |
| |
|
|
| 9.1 |
|
| |
|
SLIDE 9.1 The results from ABCSG 8 and ARNO 95 were combined for the efficacy analysis of switching from adjuvant tamoxifen to anastrozole after two years of tamoxifen in postmenopausal women with hormone-sensitive breast cancer. Results were presented at the 2004 San Antonio Breast Cancer Symposium by R Jakesz, MD.
|
| |
|
|
| 9.2 |
|
| |
|
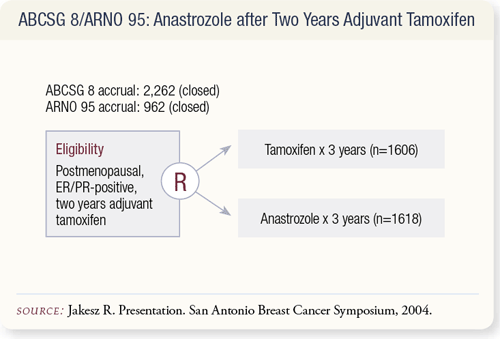
SLIDE 9.2 Both trials were initiated in 1996. In 2003, the decision was made to perform a combined analysis. The similarity in design of the two trials allowed for the combination of trial results for efficacy assessment. The majority of patients had Grade I or II tumors with favorable nodal status.
|
| |
|
|
| 9.3 |
|
| |
|
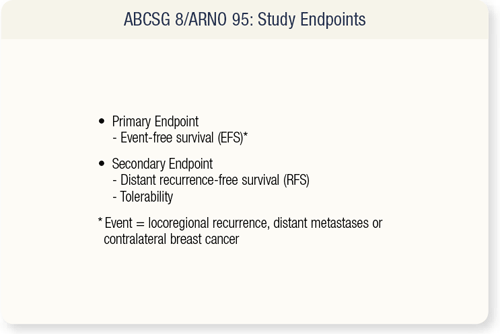
SLIDE 9.3 The primary endpoint of the study was event-free survival, and secondary endpoints included distant recurrencefree survival and tolerability. Both ABCSG 8 and ARNO 95 trials were similar in design and study endpoints to other trials of switching from tamoxifen to an aromatase inhibitor.
|
| |
|
|
| 9.4 |
|
| |
|
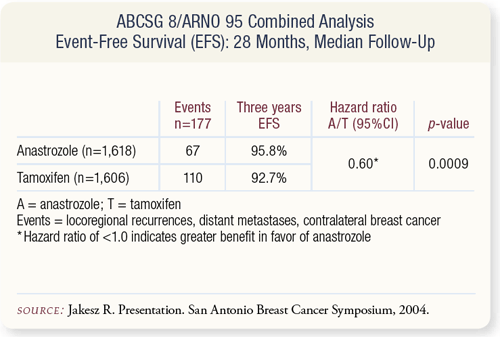
SLIDE 9.4 At a median follow-up of 28 months, there was a 40% reduction in events — which included recurrences, second breast cancers and death — in the anastrozole arm which was highly significant. A subgroup analysis of EFS showed the benefit of anastrozole occurred irrespective of age or nodal status, with greater benefit conferred on ER-positive/PR-negative tumors.
|
| |
|
|
| 9.5 |
|
| |
|
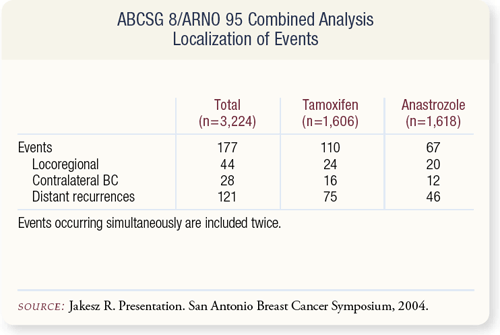
SLIDE 9.5 Most of the differences in events related to distant recurrence, which occurred in 75 women on tamoxifen and 46 on anastrozole.
|
| |
|
|
| 9.6 |
|
| |
|
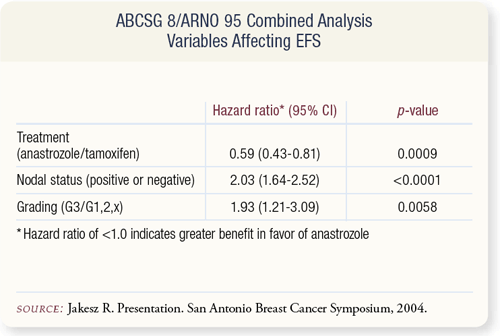
SLIDE 9.6 The benefit of switching from tamoxifen to anastrozole was independent of other prognostic factors such as nodal status and tumor grade. The anastrozole/tamoxifen hazard ratio (HR) for treatment was 0.59 with a highly significant p value of 0.0009, even though the differences due to nodal status (HR=2.03) and grade (HR=1.93) were also highly significant.
|
| |
|
|
| 9.7 |
|
| |
|
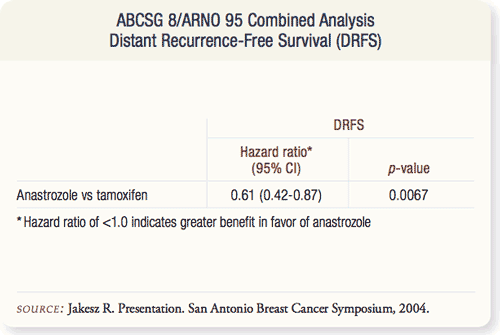
SLIDE 9.7 There was a highly significant improvement in the distant recurrence-free survival in the anastrozole arm, with a hazard ratio of 0.61 at p = 0.0087 level of significance.
|
| |
|
|
| 9.8 |
|
| |
|
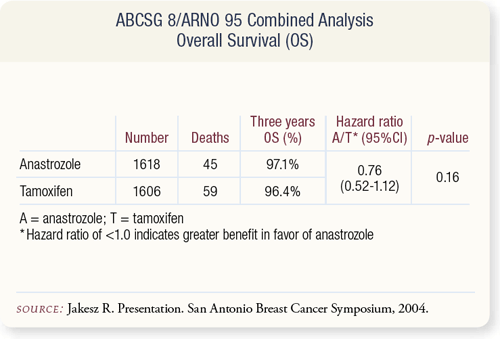
SLIDE 9.8 No difference in overall survival was observed, with only 104 deaths at the time of this analysis. However, these results are consistent with those from the ATAC trial of adjuvant anastrozole, tamoxifen or the combination (68 months of followup) and the IES trial of switching to exemestane after adjuvant tamoxifen (37.4 months of follow-up).
|
| |
|
|
| 9.9 |
|
| |
|
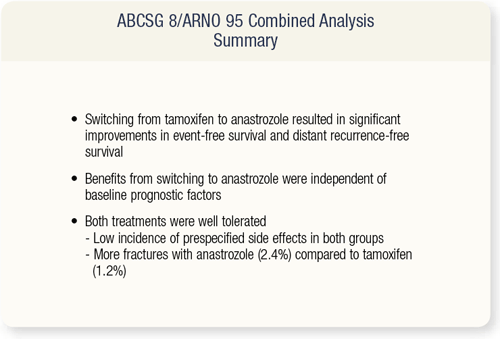
SLIDE 9.9 The outcome from three other switching trials were previously reported in 2003 and 2004. These include the ITA, IES and the NCIC-MA17 trials. All these trials reported significant improvement in disease-free survival. The results from this combined analysis provide further evidence of the benefits of switching from tamoxifen to an aromatase inhibitor.
|
| |
|
|
| |
|
Select publications |
|
|
| |
|
|
| |
|
|
| |
|
|
|
|

|
|
|

