You are here: Home: BCU 2| 2005: Michael Baum, MD, ChM
| |
|
|
| |
Michael Baum, MD, ChM |
EDITED COMMENTS |
Portions of the following were misprinted in the previous issue of BCU and are reprinted here for your convenience.
Switching postmenopausal patients from adjuvant tamoxifen to aromatase inhibitors
I am now absolutely confident that women who’ve been on tamoxifen for two or three years should switch to an aromatase inhibitor. We have excellent data for both exemestane and anastrozole from three trials. Boccardo’s small ITA trial with anastrozole was the first to report (Boccardo 2003), followed by the large IES study (Coombes 2004) with exemestane and the joint Austrian-German study of anastrozole presented in San Antonio (Jakesz 2004). Overwhelming evidence indicates that a switch to an aromatase inhibitor is beneficial.
I recommend the switch regardless of how long the patient has been on tamoxifen. You can wait forever for refinements in clinical trials, but no one is ever going to do a trial of a switch at one year or a switch at four years. We just have to stretch the available evidence and be sensible about it, and I think it would be reasonable to switch.
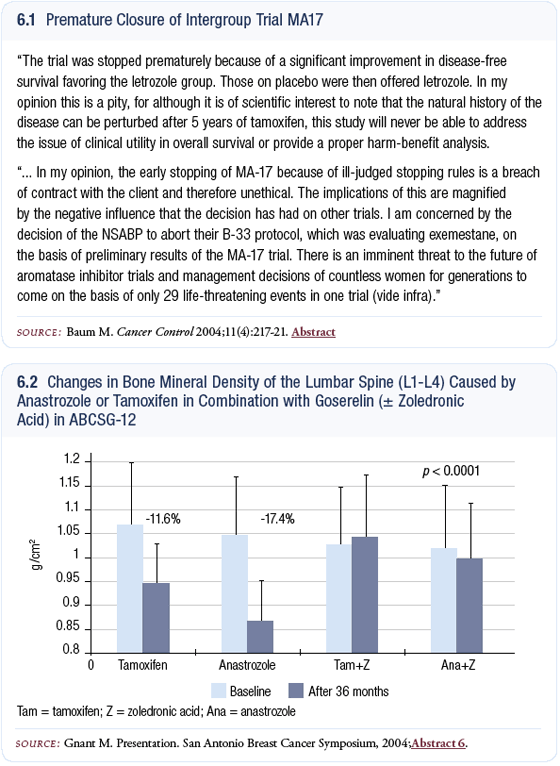
The MA17 trial is a well-conducted study (Goss 2003) in women who have already received five years of tamoxifen. It shows proof of principle that you can influence the natural history of breast cancer after five years of tamoxifen. I’ve gone on record that I’m bitterly disappointed that they closed the trial (6.1) and then allowed the placebo group to switch to letrozole, because they are treating the placebo group with experimental therapy — five years on tamoxifen, an average of two and a half years placebo, and then letrozole. That is an unproven treatment and I don’t think we’ll ever really learn the long-term benefit and toxicity.
I think we’re going way beyond the data. What worries me is that we cannot correct this situation. We’ll always be left with an area of uncertainty; however, to their eternal credit, the MA17 and NCIC group have redeemed themselves by being prepared to do a second randomization for duration after five years of the aromatase inhibitors.
Bisphosphonates in premenopausal women on tamoxifen or anastrozole
The Austrian study presented in San Antonio analyzed the capacity of zoledronic acid to prevent bone loss (Gnant 2004). The patients are all premenopausal women receiving an LHRH agonist. They are then randomly assigned to anastrozole or tamoxifen, followed by a second randomization to zoledronic acid or not.
In the main-effect analysis, zoledronic acid protects against osteopenia and osteoporosis. In the four-arm analysis, the bone mineral density in the goserelin plus anastrozole arm is the lowest, but the curve for goserelin plus anastrozole plus zoledronic acid runs parallel with the curve for goserelin plus tamoxifen plus zoledronic acid (6.2). I find that reassuring. It is evidence that zoledronic acid, a bisphosphonate, can reverse this loss of bone mineral density. The other result that was somewhat of a surprise was that even the women who received tamoxifen and goserelin were losing bone.
|
|
| |
|
|
| |
|
|
| |
|
|
|
|

|
|
|

