Maura N Dickler, MD |
EDITED COMMENTS |
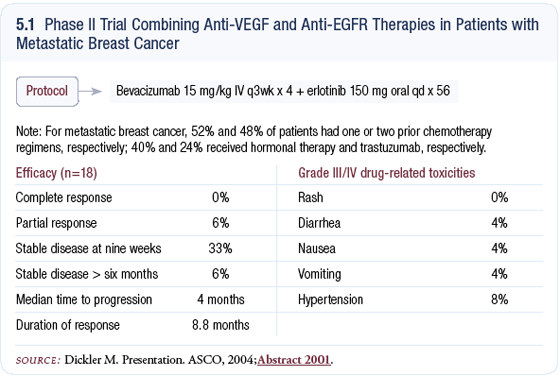
 Phase II trial of bevacizumab plus erlotinib in patients with metastatic breast cancer (18 patients) Phase II trial of bevacizumab plus erlotinib in patients with metastatic breast cancer (18 patients)
Efficacy
In the preliminary analysis, we saw one partial response, and that patient has now had approximately an 80 percent reduction in a chest wall mass and nodal metastases (Dickler 2004; [5.1]).
She’s had a very durable response for about 15 months, which is impressive. A few other patients are nearing achievement of partial responses, but currently we just have the one partial response and many patients with stable disease.
Side effects
The side effects observed are secondary to the anti-EGFR therapy, including skin rash and diarrhea. Skin rash occurs in about three-quarters of patients and can be treated in the majority of with oral tetracycline, and the diarrhea is very controllable with Imodium®.
Typically, patients can push through these side effects and improve on their own. Bevacizumab was very well tolerated, with no infusion reactions and minimal bleeding. Hypertension occurs in 15 to 20 percent of patients, but it can be well controlled with medication.
Correlative studies
We’re also looking at EGFR status, ER, PR, HER2, VEGF and VEGF receptor status. We’re also sending serial blood samples to Hope Rugo at UCSF, and in collaboration with John Park, they are looking at circulating tumor cells, circulating endothelial cells and serum angiogenic factors.
The New England Journal article demonstrated that circulating tumor cells can help predict response to therapy before CAT scans (Cristofanilli 2004). We want to determine whether changes in tumor cells and endothelial cells can potentially predict response to antiangiogenic therapy.
Rationale for evaluating trastuzumab in combination with bevacizumab
The UCLA group is studying this very interesting combination (Pegram 2004). Preclinical data recently published by Konecny and colleagues demonstrate that VEGF is upregulated in HER2-positive breast cancer (Konecny 2004), so there’s a rationale for why we believe that VEGF may be important and may partially explain the aggressive phenotype of HER2-positive breast cancer.
Management of patients with ER-positive, HER2-positive metastatic breast cancer
Off protocol, I always start with single-agent hormonal therapy if the patient has minimal symptoms from their cancer. I never want to lose an opportunity to administer an effective therapy that will also provide good quality of life. I tend to use an aromatase inhibitor, as opposed to tamoxifen, for first-line therapy for postmenopausal women. I watch patients and re-image in about three months, and if they’re progressing and I have concerns, I sometimes add trastuzumab. I have one patient who has been doing very well for approximately two years with this combined strategy.
First-line therapy for patients with ER-negative, HER2-positive disease
I look at patient’s tumor bulk, sites of disease and whether or not they’re symptomatic to determine approach to treatment. I’ve had a couple of patients who are relatively asymptomatic whom I’ve treated with trastuzumab monotherapy. Chuck Vogel’s data demonstrated that clinical benefit rates may be almost 50 percent (Vogel 2002).
In those patients receiving monotherapy, I can administer treatment every three weeks. They can continue to work; they have no alopecia or treatment-related symptoms and good quality of life. I’ve learned that metastatic breast cancer is a chronic illness, and although it is life threatening and without cure, women will live for years. It’s distressing to make patients who are asymptomatic experience the side effects of our treatments.
In patients who are symptomatic from their cancer, I utilize trastuzumab and taxanes. The benefits of those agents far outweigh the downside of the side effects. I tend to use weekly paclitaxel in combination with trastuzumab, then vinorelbine in combination with trastuzumab. I’ve also utilized the carboplatin/paclitaxel/trastuzumab triplet, and currently at my institution we are performing a pilot trial evaluating carboplatin/trastuzumab in combination with nanoparticle paclitaxel.
Solvent-free albumin-bound nanoparticle paclitaxel
Nanoparticle paclitaxel is a very interesting agent. It may be as good or better than paclitaxel, and we may have to worry less about the hypersensitivity reactions associated with paclitaxel, which is a tremendous advantage. Hypersensitivity reactions are rare, but real, and occasionally life threatening.
We can also avoid the steroid-associated toxicity, particularly when administering weekly paclitaxel to patients with metastatic disease who may receive the agent for one or even two years. Many of those women experience insulin insensitivity and diabetes, develop proximal muscle weakness and gain weight — all side effects due to the steroids.
In light of the data from Dr Blum’s trial demonstrating benefit to ABI-007 in patients with taxane-refractory metastatic breast cancer (Blum 2004; O’Shaughnessy 2004), I would be comfortable substituting nanoparticle paclitaxel for paclitaxel in patients with metastatic disease if the agent was FDA approved.* In terms of utilizing it in the adjuvant setting, particularly in a dosedense fashion, we definitely need safety data but I don’t know if we need to treat thousands of patients in an adjuvant clinical trial.
 Algorithm for selection of chemotherapy in the metastatic setting Algorithm for selection of chemotherapy in the metastatic setting
Positive data exist for several combinations, including capecitabine/docetaxel (O’Shaughnessy 2002) and paclitaxel/gemcitabine (Albain 2004). The doxorubicin/docetaxel combination improved response rate but didn’t improve overall survival, and George Sledge demonstrated that sequential therapy was as good as combination treatment in terms of overall survival (Sledge 2003), so I tend to use sequential single agents for the vast majority of my patients.
In a patient who is chemotherapy naïve and needs a rapid response, I would consider an anthracycline-based combination regimen. It would probably be doxorubicin/docetaxel, but it could also be doxorubicin/paclitaxel. If a patient had dose-dense AC/paclitaxel in the adjuvant setting, I’d be very interested in incorporating a gemcitabine-based combination or a capecitabine-based combination. I use a lot of capecitabine. I believe it’s a great drug and is generally well tolerated when given at nonpackage-insert doses.
For the patient who’s had adjuvant AC  T, I frequently use capecitabine or vinorelbine as first-line therapy. For someone who’s chemotherapy-naïve, my first choice would probably be weekly paclitaxel followed by either vinorelbine or capecitabine. I don’t use early-line doxorubicin up front very often in my asymptomatic patients, because I think it causes a lot of fatigue and alopecia. Weekly paclitaxel also results in alopecia, but I prefer to use weekly paclitaxel more than doxorubicin in the metastatic setting. T, I frequently use capecitabine or vinorelbine as first-line therapy. For someone who’s chemotherapy-naïve, my first choice would probably be weekly paclitaxel followed by either vinorelbine or capecitabine. I don’t use early-line doxorubicin up front very often in my asymptomatic patients, because I think it causes a lot of fatigue and alopecia. Weekly paclitaxel also results in alopecia, but I prefer to use weekly paclitaxel more than doxorubicin in the metastatic setting.
Selection of adjuvant endocrine therapy
Several very large, well-designed trials have evaluated aromatase inhibitors in the adjuvant setting for postmenopausal patients. The aromatase inhibitors add benefit immediately after surgery, after two to three years of tamoxifen or as extended adjuvant therapy.
In breast cancer, the highest risk of recurrence is typically within the first two to three years after surgery. In women who participated in the ATAC trial, you can see a difference in the disease-free survival curves well before the two and a half year mark (Baum 2003; Howell 2004, 2005).
Not only do you lose patients to an early breast cancer recurrence in the first two to three years, but you also lose some women to adverse events on the tamoxifen arm. The IES study (Coombes 2004) and MA17 (Goss 2003) really do not take those facts into consideration, because those women have already dropped out prior to randomization.
I typically offer anastrozole to the majority of postmenopausal patients with receptor-positive tumors after surgery and chemotherapy. When women come in after two to three years of tamoxifen, I discuss switching to an aromatase inhibitor. When women come in at the end of five years of tamoxifen, I discuss the letrozole data.
MD Anderson trial of neoadjuvant chemotherapy with or without trastuzumab
The results reported at ASCO (Buzdar 2004) were very exciting but further study is required. Approximately 40 patients were randomized on that study, and I believe the long-term toxicity data, particularly with regard to the myocardium, will be important.
Although neoadjuvant trastuzumab increased pathologic response rate — which is a promising sign that it will add to efficacy — it remains uncertain whether it will result in improved survival. I’m not using neoadjuvant trastuzumab in my patients. I would “never say never” because, for example, women with inflammatory breast cancer have a very high risk of developing distant metastatic disease. In general, at Sloan-Kettering we do not use adjuvant trastuzumab in a nonprotocol setting.
Select Publications
 |
Dr Dickler is an Assistant Attending Physician, Breast Cancer Medicine Service, at Memorial Sloan- Kettering Cancer Center in New York, New York. |
|

