Jenny C Chang, MD |
EDITED COMMENTS |
 Neoadjuvant trastuzumab trial Neoadjuvant trastuzumab trial
We looked at the activity and efficacy of neoadjuvant single-agent trastuzumab in treatment-naïve women with HER2-overexpressing, locally advanced breast cancer. We administered three weeks of single-agent trastuzumab and measured the tumor size before and after treatment.
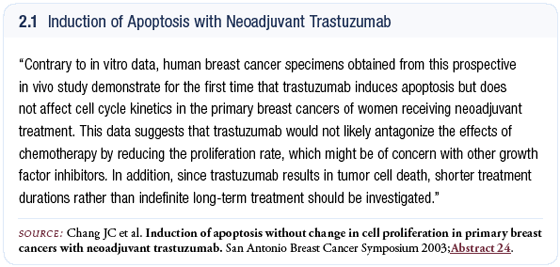
The endpoints assessed in the study were twofold: (1) efficacy and (2) the mechanism of action of trastuzumab. For the second endpoint, we looked at several pathways — proliferation, growth factor and apoptosis pathways (Chang 2003a).
We enrolled 40 patients, and after only three weeks of trastuzumab, 25 percent of the patients had a partial response (50 percent reduction). The others had stabilization of disease, and none progressed. At that point, we used four cycles of docetaxel and continued weekly trastuzumab.
All of the patients underwent surgery, and the pathologic CR rate was high — about 35 percent. The combination of docetaxel and trastuzumab appears to be synergistic and yields high pathologic CR rates, which is very encouraging. Until this study was done, cell-line and in vitro models suggested that trastuzumab was predominantly active through a decrease in proliferation. However, if it were only proliferation, you would not see such a massive reduction in tumor size. We found that the primary mechanism of action for trastuzumab is by inducing apoptosis (Chang 2003a; [2.1]).
This has important implications. Number one — trastuzumab is unlikely to be antagonistic with chemotherapy, because they both affect apoptosis, so they would more likely be synergistic.
Secondly, we might think that in studies of patients with metastatic disease — like with chemotherapy, which we use for a defined period of time — we could think about administering trastuzumab for a period of time, stopping, evaluating how the patients do then reintroducing trastuzumab in the future.
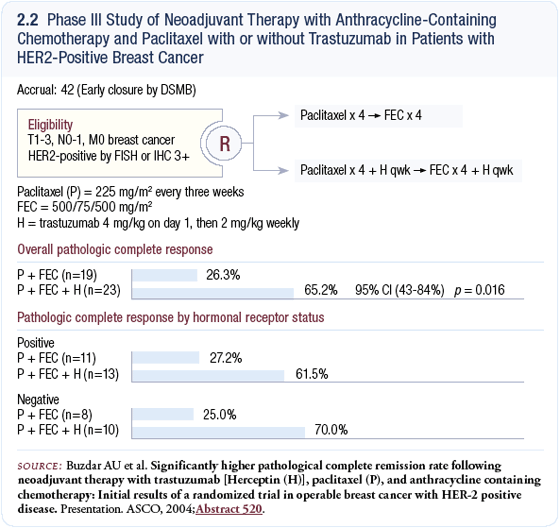
Neoadjuvant trial of trastuzumab in combination with paclitaxel and FEC
I think Dr Buzdar’s data with neoadjuvant trastuzumab in combination with paclitaxel and FEC are extremely provocative, because they demonstrate a very high pathologic complete response rate (Buzdar 2004; [2.2]). It was a small study whose results need to be confirmed by a larger study with very careful cardiac monitoring, because they administered trastuzumab together with an anthracycline, even though it was epirubicin.
I have some reservations in terms of Dr Buzdar’s cardiac monitoring data. There were no documented cases of cardiac failure, but in other studies, trastuzumab was given for a long time before the cardiac toxicity was realized. Other combinations may be as effective, like the combination of a taxane, carboplatin and trastuzumab. A taxane, trastuzumab and vinorelbine combination is synergistic also. Several regimens may circumvent the problem of cardiac toxicity associated with trastuzumab.
Pan-HER2 inhibition
I’m very interested in the concept of a pan-HER2 inhibitor. Work by Kent Osborne with a mouse xenograft indicates that complete blockade of the HER2 pathway is necessary to elicit a cure. When MCF-7/HER2 + human breast cancer cell xenografts were implanted in mice, they found that with the combination of trastuzumab, gefitinib and pertuzumab — which results in pan-HER2 blockade — tumors actually regressed completely and never came back when the combination was stopped (Arpino 2004; [2.3]). I think we’re moving into an era in which you are going to have an escape mechanism unless you block the HER family completely. If you have an escape mechanism, that could actually result in worsening of the disease.
Treatment of women with ER-negative, HER2-positive metastatic disease
In these patients, the decision to use trastuzumab alone or in combination with chemotherapy depends on the pace and bulk of the disease. If the disease is indolent and the woman has, perhaps, a skin nodule and locoregional disease, I may try single-agent trastuzumab, which buys significant quality of life. But, I do actually believe in the synergism with chemotherapy, and would add in chemotherapy shortly after.
Then comes a big question: Do you continue trastuzumab indefinitely, switching around the chemotherapies? The study that would address this has never been done. My personal bias is to continue trastuzumab. Initially, I did not do this, but I do now.
The reason is anecdotal; it’s personal experience. You see responses that you have never seen in the days before trastuzumab. The duration of response is much longer with the combination of trastuzumab plus changing around the chemotherapies. Again, that is anecdotal, and it’s not evidence based.
Neoadjuvant docetaxel trial
We recruited women with locally advanced breast cancer to define who would benefit from a neoadjuvant taxane. We obtained six core biopsies before four cycles of docetaxel 100 mg/m2 every three weeks.
After the four cycles of taxane monotherapy, we remeasured the primary cancer and compared the clinical reduction in tumor size associated with docetaxel; the patients then underwent surgery (Chang 2003b). The response rate was 60 to 70 percent, and the pathologic complete response rate was 14 to 15 percent.
From each pretreatment core biopsy, we were able to extract about three to six micrograms of RNA. Then, we performed an elaborate t-test between the women who did and those who did not respond — a “good-guy/bad-guy” case control study design — to find differentially expressed genes. We found 92 differentially expressed genes between the responders and nonresponders (Chang 2003b).
Because of the small sample size (24 patients), we were able to do another analysis known as leave-one-out cross-validation, which takes one of the samples out, reanalyzes the data and guesses the status of the sample that was removed. When we did that, we were 88 percent accurate in predicting who would respond to docetaxel. For the next eight patients who came in, we basically guessed whether they would respond, and we were right eight out of eight times (Chang 2003b).
On the basis of this Lancet paper (Chang 2003b), we have tried to establish patterns for different chemotherapy regimens. Through the Specialized Programs of Research Excellence (SPORE) mechanism, we have now almost completed a 120-patient study looking at the profiles of patients treated with docetaxel and AC. The preliminary data indicate that they are different. In the near future, we may have expression profiles that predict for response to docetaxel and AC, so we can individualize treatment for women with breast cancer.
Neoadjuvant docetaxel trial: Specific markers predicting for response
Beta-tubulin was overexpressed in tumors resistant to docetaxel. Beta-tubulin has been well documented to be involved in the mechanism of taxane resistance. On the other hand, tumors that were rapidly proliferating were more likely to be responsive to docetaxel (Chang 2003b).
Heat shock protein 27 (HSP27) overexpression is documented in doxorubicin resistance. Yet, HSP27-overexpressing cell lines remain sensitive to docetaxel. We found HSP27 was overexpressed in patients with tumors that were sensitive to docetaxel (Chang 2003b). This indicates that the patterns of gene expression for responders to docetaxel and AC — the two most commonly used regimens — are likely to be different. Therefore, we will have a tool to individualize treatments.
Select publications
 |
Dr Chang is an Associate Professor at the Breast Center at Baylor College of Medicine in Houston, Texas. |
|

