|
You are here: Home: BCU 6|2002: Melody
A Cobleigh, MD
Edited comments by Dr Cobleigh
HER2 assessment
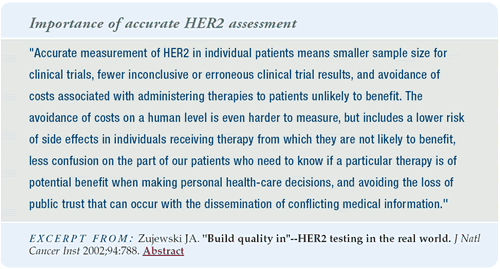
Every patient with metastatic breast cancer in my practice has
her tumor evaluated for HER2 gene amplification by FISH. Patients
with an IHC score of 3+ for HER2 overexpression should be evaluated
by FISH, because they may not have gene amplification. In those
with an IHC score of 0 or 1+ for HER2 expression, 3% and 7%, respectively,
will have HER2 gene amplification by FISH. We need to determine
HER2 status accurately, because it is a matter of life and death.
Determination of HER2 status is also important for the selection
of adjuvant chemotherapy, because patients who have the HER2 alteration
are more likely to benefit from the anthracyclines. These patients
should not be denied anthracyclines if they have a healthy heart.
Management of patients with HER2-positive metastatic
breast cancer
In patients with rapidly progressing, life-threatening, HER2-positive,
ERnegative metastatic breast cancer, I use trastuzumab in combination
with either paclitaxel or vinorelbine in women who have not previously
received a taxane. Otherwise, I use trastuzumab monotherapy.
The disease characteristics of the patients in Chuck Vogel's front-line
trastuzumab trial are very similar to those in the pivotal trial
of trastuzumab with or without chemotherapy. Both of those trials
demonstrated similar time to tumor progression, etcetera. That is
not a direct comparison, but the model that we have always used
in breast cancer is that we cannot cure metastatic disease. So,
we use the treatment that will be most likely to put the patient
in remission with the fewest side effects. Clearly, single-agent
trastuzumab is a more benign treatment than trastuzumab plus chemotherapy.
We do not yet have prospective, randomized trial data that demonstrate
a survival advantage for single-agent trastuzumab. However, if a
patient responds to trastuzumab, it will be evident very quickly,
often within a couple of weeks. If she progresses, you can always
add chemotherapy.
For patients with HER2-positive, ER-positive metastatic breast
cancer, I use tamoxifen in premenopausal women or an aromatase inhibitor
in postmenopausal women. If the patient progresses, then I would
add trastuzumab and continue hormonal therapy.
Duration of trastuzumab therapy
There are no data to guide us in what to do after a patient progresses
while receiving trastuzumab and chemotherapy. The trastuzumab story
has consistently shown that the laboratory models predict what happens
in the clinic. The laboratory models demonstrate that trastuzumab
when combined with most chemotherapeutic agents is more effective
than when a chemotherapeutic agent is used alone. Until I see a
trial that shows this is not true, I will continue trastuzumab indefinitely
along with the chemotherapy.
Nonprotocol use of adjuvant trastuzumab
I have not used adjuvant trastuzumab in a nonprotocol setting.
Our experience with bone marrow transplant taught us that we could
not always trust our preconceived notions about what would work.
We need to answer the questions regarding adjuvant trastuzumab quickly,
so I have only been entering patients — even those with high-risk,
10 or more positive nodes or inflammatory disease — on clinical
trials.
Investigating relationships between HER2 and other signaling pathways
There appears to be a relationship between the HER2 and both the
HER1 and angiogenesis pathways. ECOG has initiated a study combining
Iressa® and trastuzumab. Another trial being launched will investigate
trastuzumab in combination with an anti-VEGF. Both of these trials
will address very important questions about HER2 overexpressing
breast cancer. However, today few HER2- positive patients are trastuzumab
naïve, so completing these trials will be difficult unless
physicians enter their patients on these studies.
Molecular Assessment of Sensitivity to Herceptin
(MASH) unit
Aproblem with the earlier trastuzumab trials was that the tissue
blocks were not examined. Now, we are in a difficult situation.
The mechanisms of resistance to trastuzumab cannot be investigated
if the tumor was exposed to trastuzumab and chemotherapy, because
you cannot determine which agent caused the molecular alterations.
Many people are using trastuzumab with chemotherapy, since that
is the way it is FDA-approved for front-line therapy. So, we are
collaborating with the original trastuzumab investigators to retrieve
the tissue blocks from patients who were treated with single-agent
trastuzumab. We are also accruing new patients who are receiving
trastuzumab monotherapy to this archive.
We formed the Molecular Assessment of Sensitivity to Herceptin
(MASH) unit to create tissue arrays from these tumor specimens so
that investigators who wish to study novel ideas about the sensitivity
and resistance to trastuzumab will have the necessary material.
There is more known about the pathway now, and a rational approach
would be to look at downstream elements in the pathway to determine
if they are modified, so that doing something upstream would not
make any sense.
Clinical implications of the ATAC trial results
The ATAC trial results are provocative, and I will be delighted
to see more longterm data, particularly with regard to toxicity.
I do not intend to switch patients who are receiving tamoxifen to
anastrozole. I would consider using anastrozole de novo, in the
higher-risk, node-positive patients, but I am not yet certain whether
I would use anastrozole in node-negative patients.
There is no adjuvant data for the other aromatase inhibitors.
Right now I would only consider anastrozole, because that is the
drug that has been proven.
Neoadjuvant endocrine therapy
Data from trials of neoadjuvant endocrine therapy presented this
year are impressive and will have important implications for clinical
practice. I was impressed by Ellis' study of preoperative letrozole,
but a study using anastrozole convinced me to begin utilizing neoadjuvant
endocrine therapy. Anastrozole produced the same pathologic complete
response rate as AC followed by docetaxel in the NSABP B-27 trial.
Previously, when I encountered patients with stage IIIA/B breast
cancer, my immediate reaction was to consider which chemotherapeutic
regimen to use. Neoadjuvant endocrine therapy appears to be just
as effective as chemotherapy, and it is much more benign.
Management of patients with HER2-negative, ER-negative
metastatic breast cancer
The most depressing patient to meet is the woman with HER2-negative,
ERnegative metastatic breast cancer, where the only therapeutic
option is chemotherapy. The choice of agents depends upon what she
received previously. For patients who have not had prior chemotherapy,
I often use the old-fashion regimen, M->F, as described by the
NSABP. It does not cause alopecia, premature menopause, nausea and
vomiting or neutropenic fever. It is given day one and day eight
every four weeks, and the patient can take it indefinitely since
it is not associated with cumulative neurotoxicity. It is also inexpensive,
so M->F would likely be my choice.
For patients who had prior AC recently and relapsed, I would use
M->F or weekly paclitaxel. It is very easily tolerated, except
for the frequent visits. For patients who had ACT, there are a number
of options, but one of the easier ones for the patient is capecitabine.
It is an oral agent and only requires a visit to the doctor once
every three weeks. I am extremely impressed by the activity of capecitabine.
I have used it a lot since I am participating in the trial comparing
capecitabine with or without anti-VEGF.
Capecitabine dose reduction
I start capecitabine at a dose of 2,500 mg/m2/day, and many patients
tolerate that dose very well. However, patient education is critical.
I instruct patients to call me if they experience any changes in
their hands or feet or if they develop diarrhea. At the first sign
of toxicity, I reduce the dose by 25%. Hand-foot syndrome is only
problematic when patients do not heed warnings to call at the first
signs of symptoms. Some patients have the idea, "If I hit this
really hard, I’ll be cured." That is really a bad strategy.
Single-agent versus combination chemotherapy
for metastatic disease
Unfortunately, patients with metastatic breast cancer are routinely
getting combination chemotherapy, when sequential single-agent treatment
is just as effective in terms of survival. ECOG 1193 compared doxorubicin
(A) to paclitaxel (T) — with a crossover at progression —
to the combination (AT). There was no difference in survival, and
patients treated with AT have a worse quality of life than those
treated with sequential single-agent therapy.
I do not use combination chemotherapy in the metastatic setting,
except in patients with life-threatening disease. If the patient
has not had an anthracycline or taxane recently, I would probably
use AT. Capecitabine/docetaxel is another option for patients who
have recently progressed on an anthracycline.
Select publications
|
