|
You are here: Home: BCU 6|2002: John F. Robertson, MD, FRCS
Edited comments by Dr Robertson
Combined data from the trials of fulvestrant
versus anastrozole
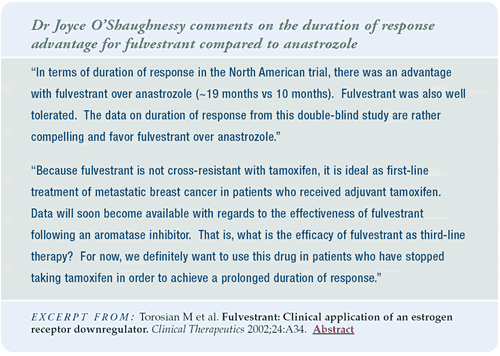
Duration of response is one of the key issues in treating metastatic
disease. In the North American trial comparing fulvestrant to anastrozole,
fulvestrant had a longer duration of response than anastrozole.
This was not seen in the European trial. However, determining the
duration of response is problematic in any study, because you are
sorting a population of patients who responded to therapy and were
not identified prerandomization.
A statistical method has been proposed for this type of analysis,
whereby nonresponders are said to have a response duration of zero.
This method looks at all of the patients in one arm compared to
another, rather than a subpopulation of responders.
Using this type of analysis on the combined data from the two
trials, fulvestrant has a longer duration of response than anastrozole
in responding patients. This is a new finding.
I would not say categorically that fulvestrant was better than
anastrozole, because this is a retrospective analysis, and we must
be statistically rigorous in interpretation. But, it does emphasize
the equivalence of these drugs in the second-line setting and gives
us more confidence to use fulvestrant. I hope that people embrace
fulvestrant, because it is a good drug with a very good sideeffect
profile.
Fulvestrant: Mechanism of action and questions
for the future
Essentially, fulvestrant prevents dimerization and increases degradation
of the estrogen receptor, which reduces the protein’s half-life.
Fulvestrant binds to the estrogen response element of the genes
and “switches off” both activation functions, AF-1 and
AF-2, which prevents translation.
Interesting questions for the future include: What would happen
with an even more potent concentration of fulvestrant or a new antiestrogen?
Is fulvestrant the best antiestrogen that we have or is there something
in development, which may completely downregulate that receptor,
so that there is no expression once you are on the drug?
Hot flashes with fulvestrant
Because fulvestrant does not cross the blood-brain barrier, I
do not think this drug causes hot flashes. It is difficult to confirm
this, however, because many women continue to get hot flashes for
years after entering the menopause. While some studies measure hot
flashes, they do not assess baseline hot flashes.
It would be important to know how many women develop new symptoms
or an increase in the severity of the symptoms. In the first-line
study, we may see some hot flashes in the fulvestrant-treated group;
however, it is possible that these symptoms were present before
treatment began.
Response to endocrine therapy after treatment
with fulvestrant
Sequencing of fulvestrant is a key issue to be addressed. We have
data that you can see responses to aromatase inhibitors after fulvestrant
and vice versa. Fulvestrant resistance is not hormone insensitivity.
We have seen responses to endocrine therapy after treatment with
fulvestrant in our own center. We had a patient who was on the first
phase II fulvestrant study. She received fulvestrant for six years
and then had a partial response to an aromatase inhibitor. At the
time she became resistant to fulvestrant, her tumor still expressed
some estrogen receptor.
We do not yet know the mechanism by which cancer becomes resistant
to fulvestrant. We do not believe it is an agonistic property. As
in this case, we can see ER expression in patients on fulvestrant
— even at the time of resistance. We are studying mechanisms
of resistance with sequential biopsies to examine the specimens
both when the patients are responding and resistant.
Fulvestrant in premenopausal women
Fulvestrant’s affinity for the estrogen receptor is roughly
equivalent to that of estradiol. Because premenopausal women have
such high levels of estradiol, we do not know if the degree of estrogen
receptor downregulation will be equivalent to that in the postmenopausal
woman.
There is one study in premenopausal women comparing preoperative
fulvestrant to placebo. The participants underwent biopsies for
diagnosis and were then given 250 mg of fulvestrant or placebo,
two to three weeks before surgery. Another specimen was removed
at the time of surgery to assess the presence of ER and PGR as well
as the degree of downregulation.
My guess is that we will see the same or slightly less downregulation
in premenopausal women compared to postmenopausal patients. Hopefully,
we will have the answer by the end of the year.
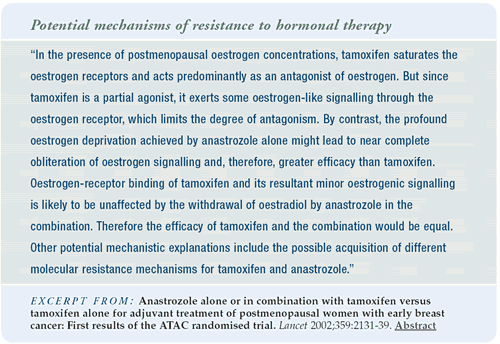
Interpreting the ATAC trial results
I predicted that at this early analysis the ATAC trial would show
no difference between the treatments. But in fact, the trial does
show a statistically significant improvement in disease-free survival
with anastrozole compared to tamoxifen alone or the combination
of anastrozole plus tamoxifen. Anastrozole was also better tolerated
in terms of hot flashes, cerebrovascular accidents, DVTs, vaginal
dryness, vaginal discharge and endometrial cancer. Tamoxifen was
better tolerated with regard to musculoskeletal symptoms (i.e.,
joint pain) and bone fractures.
There are several reasons to think these results are true. This
is the largest adjuvant endocrine study ever conducted, with 9,366
patients. When the ATAC trial started, the largest trials to that
point were the CRC study and the NSABP B-14 tamoxifen study, which
had less than 3,000 patients each. Given the size of the ATAC trial,
it is unlikely that the results are a chance event.
The consistency of the data internally and the data from the metastatic
setting also gives us confidence. In the ATAC trial, there were
fewer local and distant recurrences and contralateral breast cancers
in the patients treated with anastrozole compared to those treated
with tamoxifen. As first-line therapy for metastatic disease, anastrozole
has a time to progression benefit in hormone receptor-positive patients
compared to tamoxifen (ten versus six months). The Spanish Milla-Santos
study with anastrozole and studies with other aromatase inhibitors
in the metastatic setting similarly found an advantage over tamoxifen.
Now we are seeing this additional benefit in the adjuvant setting.
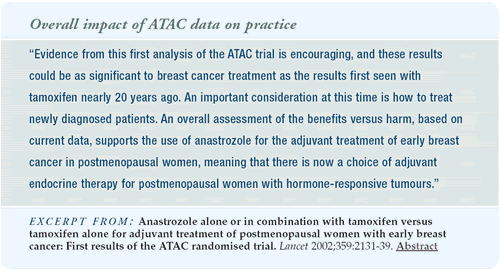
Clinical management in light of the ATAC trial
results
Should we put our postmenopausal patients on adjuvant anastrozole
now? The efficacy data is pretty secure, and the curves are diverging.
With tamoxifen, we see carryover effects even after the drug is
stopped. I suspect the curves will continue to diverge with anastrozole
as well and that the efficacy advantage will eventually transfer
into a survival benefit.
There is a spectrum of responses to this data. Very cautious clinicians
opt to use tamoxifen until we have long-term data. The ATAC trial
results do, however, reassure these individuals that patients who
are not good candidates for tamoxifen (i.e., history of thromboembolic
disease or cerebrovascular accidents) can be given anastrozole.
Many physicians already do this off-label, but the data gives us
more confidence to possibly lower the threshold to use anastrozole.
Other physicians believe that the efficacy data from the ATAC
trial is sufficient to use adjuvant anastrozole at the present time.
By the time patients have been on anastrozole for one to five years,
we will have far more data to make decisions. These physicians will
embrace the efficacy data and accept the possibility of the unknown
long-term effects on bone mineral density, or they will treat patients
prospectively with a bisphosphonate.
Side-effect profile of anastrozole compared to
tamoxifen
In many cases, anastrozole has a better side-effect profile than
tamoxifen. Although there were more fractures in the patients on
anastrozole, there were less thromboembolic events, hot flashes
and endometrial cancers. In addition, while some of tamoxifen’s
side effects are manageable, they are usually not preventable.
In contrast, anastrozole’s main side effect — bone
fractures — can potentially be prevented with bisphosphonates,
calcium supplements or exercise. Some clinicians would rather take
the risk to gain better efficacy, and they may elect to start patients
on a bisphosphonate to hopefully prevent bone mineral density loss.
The large number of patients in the ATAC trial gives us confidence
that there are not any serious but uncommon side effects associated
with anastrozole.
Discussing anastrozole with patients
I believe the ATAC trial data should be discussed with any postmenopausal
woman starting tamoxifen. If the issues are explained clearly, most
women are able to understand and voice their opinion on these matters.
This is one of the largest adjuvant studies ever done, and ignoring
the data is a disservice to women.
The ATAC trial, however, does not answer all the questions. It
simply tells us that anastrozole appears to be more active than
tamoxifen. There are unknowns in terms of the effects on bone mineral
density, sequencing, duration and interactions with chemotherapy.
Patients should receive all of the information and make decisions
based on these unknowns.
Substituting other aromatase inhibitors for anastrozole
I do not think we should use the other aromatase inhibitors —
letrozole and exemestane — as adjuvant therapy. We have to
wait for the adjuvant studies with these agents. We cannot extrapolate
that drugs with similar efficacy in advanced disease will be exactly
the same in the adjuvant setting, either in terms of efficacy or
side-effect profile.
The degree of aromatase inhibition is slightly different between
the agents. There have been claims that letrozole reduces estrogen
levels fractionally more than the other aromatase inhibitors. While
this difference may or may not translate into an efficacy benefit,
there are two sides to every sword — it also may translate
into a worse side-effect profile. For example, the little bit of
estrogen remaining in a woman’s body with anastrozole may
provide some protection in terms of bone mineral density loss and
bone fractures. My personal view is that the differences we will
see between the aromatase inhibitors in the adjuvant setting will
most likely be in terms of their side-effect profiles rather than
efficacy.
Select publications
|
