|
You are here: Home: BCU 6|2002: Joyce O'Shaughnessy,
MD
Edited comments by Dr O'Shaughnessy
Capecitabine/docetaxel (XT) versus docetaxel
alone in metastatic disease
Follow-up of survival data
The trial comparing capecitabine/docetaxel to docetaxel
alone is mature now, and the combination is definitely superior
in terms of overall survival. Median survival for capecitabine/
docetaxel is 14.5 months compared to 11.5 months for docetaxel.
At the 12-month mark, 57% of the patients receiving the combination
were alive compared to 47% of those randomized to docetaxel alone.
There were few treatment-related deaths in both arms, and the rates
of hospitalization and serious adverse events were similar between
the two regimens.
Analysis of post-study therapy
Currently, there is a question about whether to use sequential
single agents or the capecitabine/docetaxel combination. David Miles
from the UK did an analysis of post-trial therapy. Approximately
two-thirds of the patients in both arms received chemotherapy after
disease progression, and 27% of the patients who received chemotherapy
after progressing on docetaxel received capecitabine.
The hazard ratio for mortality in the patients who received capecitabine
after docetaxel compared to any other chemotherapy agent was 0.5
— a 50% reduction in the risk of dying. In the group who received
vinorelbine after docetaxel, the hazard ratio for mortality compared
to any other chemotherapy excluding capecitabine was 1.0. This data
gives some credence to the folks who have been saying that it is
okay to give single-agent docetaxel followed by capecitabine. The
way I interpret the data from a conservative standpoint is in patients
with relatively asymptomatic indolent disease, it is very reasonable
to give docetaxel and capecitabine sequentially.
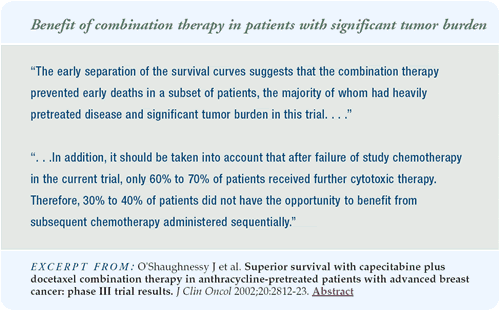
Conversely, given the early separation of the survival curves
and the early death rate with docetaxel alone, there is a subgroup
of patients with more aggressive, symptomatic disease who will not
have the opportunity to receive sequential therapy. For these patients,
the capecitabine/docetaxel combination may be preferred.
There is also a hypothesis, which cannot be addressed by these
data, that a trial comparing capecitabine/docetaxel to docetaxel
followed by capecitabine would still result in a survival advantage
for the combination. The combination has a very clear biochemical
and preclinical synergy, which is quite different from most other
doublets. Docetaxel upregulates thymidine phosphorylase, which leads
to the enhanced conversion of the capecitabine prodrug to 5-FU at
the tumor site.
Quality of life
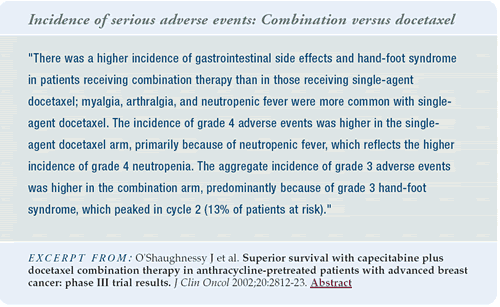
From an acute toxicity standpoint, the capecitabine/docetaxel
combination has more toxicity than docetaxel alone. Docetaxel results
in more febrile neutropenia, because it is more myelosuppressive.
But, the capecitabine/docetaxel regimen results in more diarrhea
and hand-foot syndrome.
A careful quality-of-life analysis was done in this large phase
III trial. For the first four to five months of the study, the curves
overlap. Afterwards they separate with capecitabine/docetaxel being
superior to docetaxel alone, clearly as a result of better tumor
control with the combination. The deterioration in the docetaxel
arm is undoubtedly due to tumor progression. These patients were
heavily tumor-burdened and intensively pretreated. Two-thirds of
them received the study therapy as second- or third-line therapy,
so these patients were fairly ill.
Oral versus intravenous chemotherapy for metastatic
disease
Patients who relapse after adjuvant therapy are scared to death,
and most of them are still in the "fight mode" at that
point. If a patient wants the most effective therapy, I will recommend
combination chemotherapy. However, many older women with very indolent
disease who have undergone treatment for a long time consider their
quality of life to be very important. For these patients, being
treated with a very effective pill is attractive.
Phase II trials comparing capecitabine to paclitaxel
or CMF in patients with metastatic disease
Capecitabine versus paclitaxel
Capecitabine is quite an active compound. A small randomized
phase II trial in anthracycline-pretreated patients comparing capecitabine
to paclitaxel 175 mg/m2 every three weeks was stopped prematurely,
because it was difficult to randomize patients to either a pill
or an intravenous treatment. The response rate with capecitabine
was 36% compared to 26% with paclitaxel with widely overlapping
confidence intervals — they were basically equivalent.
Capecitabine versus CMF
We conducted a larger study comparing capecitabine to CMF
in elderly patients as front-line therapy. The response rate to
capecitabine was 30% compared to 16% for CMF. Approximately one-half
of those patients received prior adjuvant therapy, so they were
not all anthracycline-pretreated. Docetaxel has a solid 30% response
rate in anthracycline-pretreated patients, so it is possible that
capecitabine is close to equivalent to docetaxel.
Proposed NSABP neoadjuvant capecitabine/docetaxel
trial
NSABP B-27 was a three-arm neoadjuvant trial for operable breast
cancer — either clinically node-negative or node-positive
— which randomized patients to preoperative AC followed by
surgery, preoperative AC followed by preoperative docetaxel and
then surgery or preoperative AC followed by surgery and then postoperative
docetaxel.
The study demonstrated a doubling of the pathologic complete response
rate with preoperative AC followed by preoperative docetaxel compared
to preoperative AC alone. Disease-free or overall survival data
is not yet available, but the doubling of pathologic complete response
rate in both ER/PR-negative and ER/PR-positive patients is impressive
and a key point for clinicians.
The NSABP will make that the standard arm of their next clinical
trial — preoperative AC for four cycles followed by preoperative
docetaxel for four cycles — and the investigational arm will
be preoperative AC x 4 followed by docetaxel/capecitabine for four
cycles.
US Oncology adjuvant capecitabine/docetaxel trial
US Oncology will conduct a clinical trial in node-positive or
high-risk nodenegative, ER/PR-negative or ER/PR-positive patients
comparing adjuvant AC followed by capecitabine/docetaxel or docetaxel
alone. The doses in the combination arm will be capecitabine 950
mg/m2 (B.I.D. for two weeks, then one week off), which is 1,900
mg/m2/day and docetaxel 75 mg/m2. This represents a 25% dose reduction
for capecitabine — down from the standard dose of 2,500 mg/m2/day.
This is appropriate, because there have been extensive analyses
of the effectiveness of capecitabine dose reductions.
In our phase III metastatic trial, the median delivered dose intensity
of capecitabine in the combination arm was 75% of the intended dose,
and most patients were dose-reduced by the second cycle of therapy.
That dose was maintained for the rest of the study, and a survival
advantage still occurred in the capecitabine/docetaxel arm.
Adjuvant taxanes in ER/PR-positive patients
In both the NSABP B-28 and the CALGB 9344 trials of adjuvant AC
followed by paclitaxel, subset analyses demonstrated a very interesting
and clinically significant trend toward an improved hazard ratio
for mortality in ER/PRnegative patients but not in ER/PR-positive
patients. I have been somewhat puzzled by those findings.
The NSABP recently reported the results of their preoperative
study. In NSABP B-27, the pathologic complete response rate doubled
in ER/PRnegative patients from 13% with AC compared to 25% with
AC followed by docetaxel. In patients with ER/PR-positive tumors
there was also a definite benefit, with complete pathologic response
rates improving from 5-6% to 13- 14% with the addition of the taxane
to AC preoperatively. There is not yet disease-free and overall
survival data, so we have to be cautious, but I view this as a rationale
to use preoperative AC followed by docetaxel in higherrisk patients,
even if they are ER/PR-positive.
Potential synergy of trastuzumab/gemcitabine
In 1999, we initiated a phase II trial of gemcitabine/trastuzumab
in women with HER2 overexpressing (IHC 2+ or 3+) metastatic breast
cancer. The patients had a median of three prior chemotherapy regimens,
and almost all had received anthracyclines and taxanes. The study
regimen consisted of gemcitabine 1,200 mg/m2 on day one and day
eight in a 21-day cycle and standard weekly trastuzumab. The overall
response rate was 37%. Two-thirds of the patients scored 3+ on IHC
for HER2 overexpression, and in that subset the response rate was
45%.
Interestingly, in Melody Cobleigh’s phase II trial, patients
received singleagent trastuzumab as second- or third-line therapy,
and there was a 15% response rate. Single-agent gemcitabine after
anthracyclines and taxanes has demonstrated response rates ranging
from about 12% to 20%. So, in our study of a very heavily pretreated
group of patients, the 45% response rate with the combination of
gemcitabine and trastuzumab suggests that there may be synergy or
at least additivity between these agents.
Kevin Fox at the Fox-Chase Cancer Center is conducting a study
with gemcitabine and trastuzumab as first-line treatment for metastatic
breast cancer to see if the combination will produce the 70% to
80% response rates observed with vinorelbine, docetaxel and weekly
paclitaxel. It was very well tolerated, no unexpected cardiac effects
and no unexpected toxicities.
Duration of trastuzumab therapy
I was really impressed with the original trastuzumab pivotal trial.
Some patients had stable remissions on paclitaxel and trastuzumab
that lasted for years. If my patients are doing well on trastuzumab
plus any chemotherapy, I do not stop therapy until progression.
However, on a daily basis, we are presented with patients with
metastatic disease who have progressed on trastuzumab. There are
no data to guide us in managing these patients. I will usually continue
trastuzumab and add another chemotherapy agent. Trastuzumab is very
well tolerated, and you are not really causing harm to the patient
by continuing it. Several agents have shown remarkable activity
in combination with trastuzumab. We are consistently seeing very
high antitumor activity with paclitaxel, docetaxel, vinorelbine
and possibly gemcitabine. We are trying to milk all the activity
we can get out of these agents.
Currently, I am caring for a woman who was treated with docetaxel,
vinorelbine and rastuzumab in a phase II clinical trial. She had
a fantastic, long-term durable response for lung metastases. Subsequently,
she developed slow, progressive disease. I treated her with capecitabine,
to which she responded. After progression, she went on to receive
an investigational antifolate agent, pemetrexed, but she did not
respond. She never had paclitaxel, so I treated her with weekly
paclitaxel and trastuzumab. She has been in remission for two and
a half years, even though she had already received trastuzumab.
Perhaps she would have responded to paclitaxel alone, but my instinct
is that the combination is an effective regimen for her. In the
absence of definitive data, we use our clinical intuition in these
situations.
Dr O'Shaughnessy comments on Miami Patterns of
Care survey results
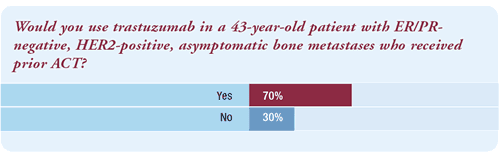
“It is not surprising that only 70% of oncologists would
use first-line trastuzumab for a patient with metastatic disease.
It is not widely appreciated, but if you read Slamon's paper closely,
the maximal survival advantage from trastuzumab is from using it
up front. The study was actually a crossover study in which two-thirds
of patients eventually received trastuzumab.”
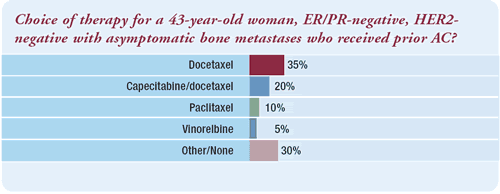
“The choice of therapy for such a patient would depend,
in part, upon her diseasefree interval. If she relapsed quickly
with a lot of bony disease — even if she was asymptomatic
— I would recommend capecitabine/docetaxel. Conversely, if
her disease was minimal, then I would consider capecitabine alone
or either weekly paclitaxel or docetaxel.”
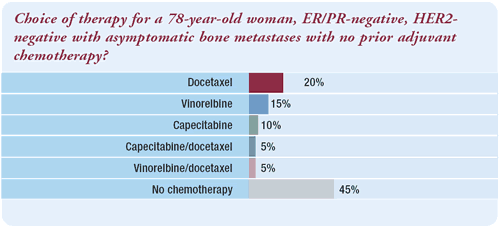
“There are a wide variety of single agents — with
similar efficacy — from which to choose. Quality of life is
an important consideration, and I would use either low dose capecitabine
or vinorelbine. In this setting, we look for efficacy and quality
of life — issues such as avoiding hair loss are important.
Docetaxel is the only single-agent therapy that has a survival advantage
in anthracycline pretreated patients; however, this woman is not
anthracycline pretreated.”
Select Publications
|
