
| Tracks 1-3 |
| Track 1 |
EFECT: Equivalence of fulvestrant and exemestane as second-line or later therapy |
| Track 2 |
Patient preferences for oral versus parenteral therapy |
|
| Track 3 |
Clinical trial strategies evaluating
fulvestrant in combination with
the aromatase inhibitors |
|
|
Select Excerpts from the Discussion
Track 1
 DR LOVE: Paul, what are your thoughts about the efficacy of fulvestrant
and the use of a loading dose? DR LOVE: Paul, what are your thoughts about the efficacy of fulvestrant
and the use of a loading dose? |
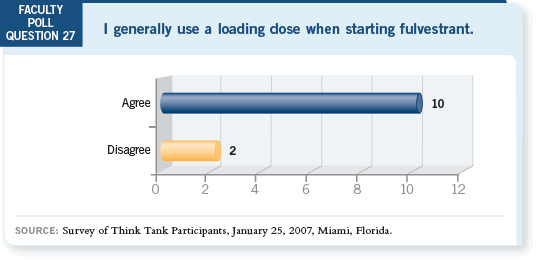
 DR GOSS: Using a loading dose seems like the appropiate way to administer
fulvestrant. Many people believe fulvestrant’s pharmacology has impaired its
activity. Several studies are being conducted that will provide additional data
on this topic.
DR GOSS: Using a loading dose seems like the appropiate way to administer
fulvestrant. Many people believe fulvestrant’s pharmacology has impaired its
activity. Several studies are being conducted that will provide additional data
on this topic.
One strategy in metastatic disease is evaluating a loading dose and then
maintaining a high dose of fulvestrant. A neoadjuvant trial, called the
NEWEST trial, is comparing a loading dose to a standard dose.
In that trial, an early rebiopsy will examine cell proliferation and other
markers in the tumor to determine whether the loading dose has a biologic
effect. I don’t believe we have evidence that fulvestrant is better than an
aromatase inhibitor as initial therapy for metastatic disease.
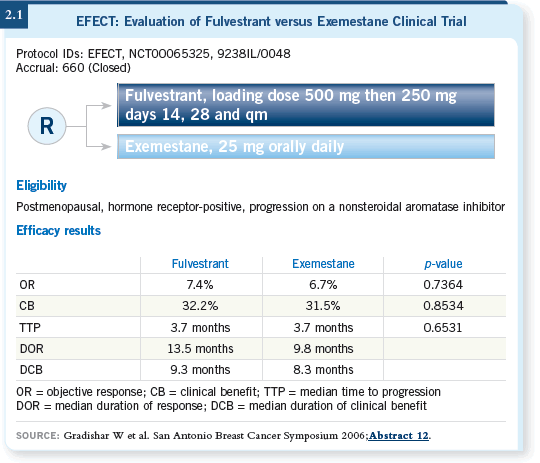
However, I believe the results from EFECT (Evaluation of Fulvestrant versus
Exemestane Clinical Trial) are convincing that fulvestrant and exemestane are
comparable for patients who have failed on a nonsteroidal aromatase inhibitor
(Gradishar 2006; [2.1]).
 DR WINER: We’re all using a loading dose of fulvestrant. However, zero data
are available regarding its efficacy because it wasn’t compared to a standard
dose.
DR WINER: We’re all using a loading dose of fulvestrant. However, zero data
are available regarding its efficacy because it wasn’t compared to a standard
dose.
It is interesting that we’ve had a number of discussions about wanting data from
Phase III randomized studies before writing a prescription, and in this case, the
pharmacokinetic data are on slides and we have no efficacy data. However, 10
out of 12 of us are using a loading dose, including myself.
Track 2
 DR LOVE: Paul, can you comment on the results of the EFECT study? DR LOVE: Paul, can you comment on the results of the EFECT study? |
 DR GOSS: I’m always skeptical about stable disease in chronic endocrine-responsive
disease, but if it’s true that about 35 percent of patients benefit from
exemestane following failure with letrozole or anastrozole (Gradishar 2006),
it implies a substantial lack of cross resistance between those two classes of
aromatase inhibitors.
DR GOSS: I’m always skeptical about stable disease in chronic endocrine-responsive
disease, but if it’s true that about 35 percent of patients benefit from
exemestane following failure with letrozole or anastrozole (Gradishar 2006),
it implies a substantial lack of cross resistance between those two classes of
aromatase inhibitors.
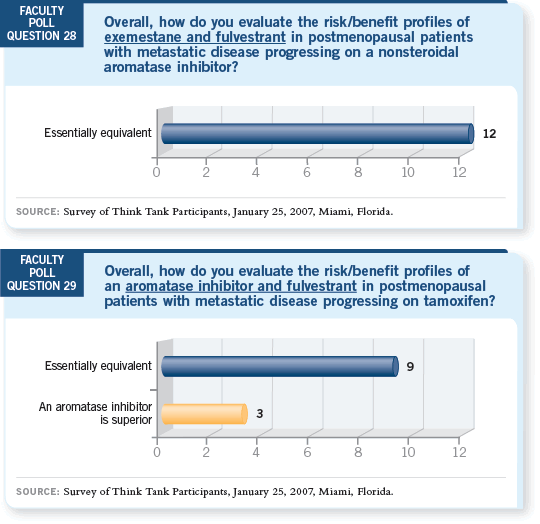
 DR SPARANO: In the Phase III trials with patients whose disease progressed
on tamoxifen (Robertson 2003) and patients whose disease progressed on an
aromatase inhibitor (Gradishar 2006), the treatment arms (fulvestrant versus
anastrozole and fulvestrant versus exemestane, respectively) produced comparable
results. I still believe the aromatase inhibitor in both cases is a winner
because the average patient would prefer to receive an oral agent.
DR SPARANO: In the Phase III trials with patients whose disease progressed
on tamoxifen (Robertson 2003) and patients whose disease progressed on an
aromatase inhibitor (Gradishar 2006), the treatment arms (fulvestrant versus
anastrozole and fulvestrant versus exemestane, respectively) produced comparable
results. I still believe the aromatase inhibitor in both cases is a winner
because the average patient would prefer to receive an oral agent.
Second, we have a fair amount of information now regarding the efficacy of
fulvestrant following an aromatase inhibitor, whereas we don’t have that information
for the converse. For those reasons, most people still choose an aromatase
inhibitor and reserve fulvestrant in the event of progression.
Track 3
 DR LOVE: Kathy, where is fulvestrant heading in terms of ongoing and
future clinical trials? DR LOVE: Kathy, where is fulvestrant heading in terms of ongoing and
future clinical trials? |
 DR PRITCHARD: Ongoing trials are comparing an aromatase inhibitor with
fulvestrant to an aromatase inhibitor alone in the metastatic setting. An evaluation
of that same question in the adjuvant setting has been proposed.
DR PRITCHARD: Ongoing trials are comparing an aromatase inhibitor with
fulvestrant to an aromatase inhibitor alone in the metastatic setting. An evaluation
of that same question in the adjuvant setting has been proposed.
 DR LOVE: Paul, what do you think about the strategy of combining an
aromatase inhibitor and fulvestrant?
DR LOVE: Paul, what do you think about the strategy of combining an
aromatase inhibitor and fulvestrant?
 DR GOSS: I like that strategy. I’ve said many times that I believe this type of
trial is one of the pieces of an important puzzle about endocrine therapy. We
need to continue pursuing the optimization of standard endocrine therapy.
DR GOSS: I like that strategy. I’ve said many times that I believe this type of
trial is one of the pieces of an important puzzle about endocrine therapy. We
need to continue pursuing the optimization of standard endocrine therapy.
 DR LOVE: In a clinical setting, for patients with metastatic disease progressing
on an aromatase inhibitor, are there situations in which you would continue
the aromatase inhibitor and add fulvestrant?
DR LOVE: In a clinical setting, for patients with metastatic disease progressing
on an aromatase inhibitor, are there situations in which you would continue
the aromatase inhibitor and add fulvestrant?
 DR GOSS: I do occasionally, but I talk to the patient carefully about it. I do it
because I often wonder how many more endocrine therapies I have left to try
with this patient. I can see chemotherapy approaching on the horizon, and I
believe perhaps I have one more crack.
DR GOSS: I do occasionally, but I talk to the patient carefully about it. I do it
because I often wonder how many more endocrine therapies I have left to try
with this patient. I can see chemotherapy approaching on the horizon, and I
believe perhaps I have one more crack.
After progression on an aromatase inhibitor, we don’t have a standard therapy.
It’s dealer’s choice whether to switch to another hormone therapy or to add
fulvestrant to an aromatase inhibitor.
Select Publications

