
| Tracks 1-10 |
| Track 1 |
Delayed adjuvant therapy with aromatase inhibitors |
| Track 2 |
Defining a time limit for the benefit of delayed adjuvant endocrine interventions |
| Track 3 |
Compliance with oral endocrine therapy |
| Track 4 |
Natural history of node-negative
and node-positive, hormone
receptor-positive disease |
| Track 5 |
Extended adjuvant therapy with aromatase inhibitors beyond five years |
|
| Track 6 |
Long-term estrogen deprivation and neuropsychiatric function |
| Track 7 |
Clinical approach to patients who
have received five years of an
adjuvant aromatase inhibitor |
| Track 8 |
HER2 as a marker of relative resistance to endocrine therapy |
| Track 9 |
Selection of initial endocrine therapy in postmenopausal patients |
| Track 10 |
Up-front use of adjuvant aromatase inhibitors versus sequencing after tamoxifen |
|
|
Select Excerpts from the Discussion
Track 1
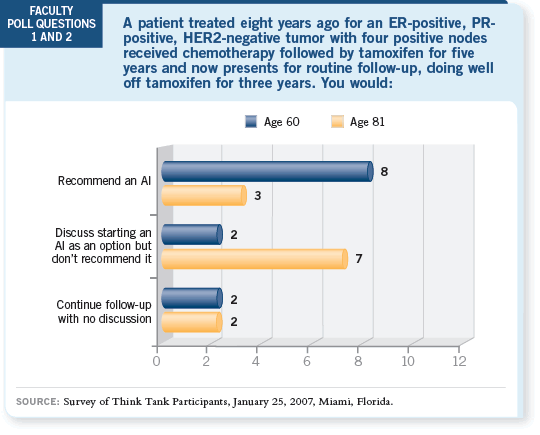
 DR LOVE: Kathy, would you recommend delayed endocrine therapy in
this situation? DR LOVE: Kathy, would you recommend delayed endocrine therapy in
this situation? |
 DR PRITCHARD: For reasons I don’t understand, data from MA17 are now
showing that the women who were initially on placebo and crossed over to
letrozole after the trial stopped have actually done better than the women who
were originally assigned to placebo. That’s not a randomized comparison, but
I believe it gives us a clear signal that starting an aromatase inhibitor even one,
two or three years after finishing tamoxifen still has an effect.
DR PRITCHARD: For reasons I don’t understand, data from MA17 are now
showing that the women who were initially on placebo and crossed over to
letrozole after the trial stopped have actually done better than the women who
were originally assigned to placebo. That’s not a randomized comparison, but
I believe it gives us a clear signal that starting an aromatase inhibitor even one,
two or three years after finishing tamoxifen still has an effect.
I believe using an aromatase inhibitor for either of these patients is logical, and
I probably would recommend one in both cases. The question with the older
patient is, how long does the average 81-year-old live? Still, she’s at high risk
and Muss’s paper suggests that older women don’t experience many quality-of-life
problems on aromatase inhibitors, at least in the short term (Muss 2006).
 DR GOSS: I feel strongly that we have a large, prevalent pool of women in the
world with hormone receptor-positive breast cancer and that many of them
have been incompletely treated. We’re seeing a benefit across a huge spectrum
of time for the application of endocrine therapy, and I believe clinicians should
ask, “Why shouldn’t I use it?” rather than, “Why should I use it?”
DR GOSS: I feel strongly that we have a large, prevalent pool of women in the
world with hormone receptor-positive breast cancer and that many of them
have been incompletely treated. We’re seeing a benefit across a huge spectrum
of time for the application of endocrine therapy, and I believe clinicians should
ask, “Why shouldn’t I use it?” rather than, “Why should I use it?”
I see patients being excluded unnecessarily based on age, preexisting osteoarthritis
and other trivial reasons. Clinicians are dismissing the application of a
treatment that’s highly effective, and I believe this problem must be addressed
by the oncology community worldwide.
 DR LOVE: Is there an upper limit, in terms of the number of years since
completing tamoxifen, at which you feel it’s been too long to consider
additional adjuvant endocrine therapy?
DR LOVE: Is there an upper limit, in terms of the number of years since
completing tamoxifen, at which you feel it’s been too long to consider
additional adjuvant endocrine therapy?
 DR GOSS: The data are confined to a range of one to seven years, from our
postunblinding analysis. It was previously shown, biologically, that if you
initiate tamoxifen at any time in the pathway of this follow-up, you can effect
benefit, and I believe that’s true here.
DR GOSS: The data are confined to a range of one to seven years, from our
postunblinding analysis. It was previously shown, biologically, that if you
initiate tamoxifen at any time in the pathway of this follow-up, you can effect
benefit, and I believe that’s true here.
Tracks 2, 5
 DR LOVE: Matt, when you see a postmenopausal patient who was
diagnosed 10 or 15 years ago and never received adjuvant endocrine
therapy, do you consider therapy now? DR LOVE: Matt, when you see a postmenopausal patient who was
diagnosed 10 or 15 years ago and never received adjuvant endocrine
therapy, do you consider therapy now? |
 DR ELLIS: That presents a conundrum. I’m certain Paul would agree that a
point exists at which offering endocrine therapy is inappropriate. One would
imagine that it could be as far out as 15 to 20 years. Certainly the idea that patients with ER-positive disease have a poorer prognosis beyond five years
underscores the fact that intervention could be beneficial.
DR ELLIS: That presents a conundrum. I’m certain Paul would agree that a
point exists at which offering endocrine therapy is inappropriate. One would
imagine that it could be as far out as 15 to 20 years. Certainly the idea that patients with ER-positive disease have a poorer prognosis beyond five years
underscores the fact that intervention could be beneficial.
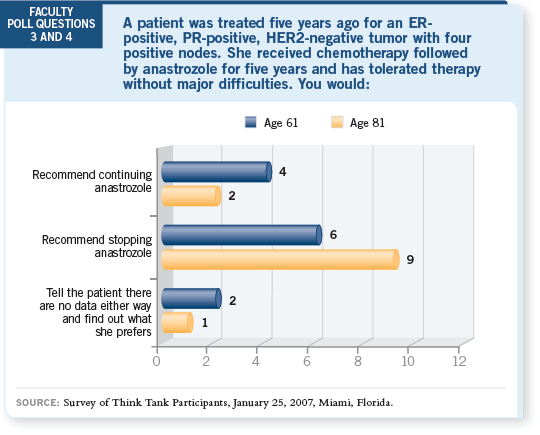
 DR WINER: I don’t object to administering an aromatase inhibitor to the
elderly woman eight years after tamoxifen, and I would agree that her median
survival is probably in the range of six to seven years.
DR WINER: I don’t object to administering an aromatase inhibitor to the
elderly woman eight years after tamoxifen, and I would agree that her median
survival is probably in the range of six to seven years.
However, it’s extremely unlikely that a modeling approach or clinical trial
would demonstrate a survival advantage here. I expect the disease-free survival
advantage will be modest for an 81-year-old patient, depending on her
competing morbidities, so I’m not going to push her to receive an aromatase
inhibitor at this point.
 DR LOVE: Aman, how much of a delay are you comfortable with?
DR LOVE: Aman, how much of a delay are you comfortable with?
 DR BUZDAR: I’m comfortable within the MA17 period, which was approximately
six months. Although our gut reaction is that we should be prescribing
endocrine therapy to these patients, currently we don’t have strong evidence to
support that, except for the MA17 data, which came after code breaking and the
patients were given a choice — they were not randomly assigned.
DR BUZDAR: I’m comfortable within the MA17 period, which was approximately
six months. Although our gut reaction is that we should be prescribing
endocrine therapy to these patients, currently we don’t have strong evidence to
support that, except for the MA17 data, which came after code breaking and the
patients were given a choice — they were not randomly assigned.
I believe we need to wait until we have prospectively randomized studies
before we offer all patients delayed therapy.
 DR GOSS: Although I agree that a randomized trial would be preferable,
it’ll be many years before we have such data, and it’s difficult to imagine that
with the strong biological effect we’re seeing in MA17 — albeit not Level 1
evidence — there’s no likelihood of affecting events for patients with hormone
receptor-positive disease in follow-up.
DR GOSS: Although I agree that a randomized trial would be preferable,
it’ll be many years before we have such data, and it’s difficult to imagine that
with the strong biological effect we’re seeing in MA17 — albeit not Level 1
evidence — there’s no likelihood of affecting events for patients with hormone
receptor-positive disease in follow-up.
Tracks 6-7
 DR LOVE: In the clinical setting, how do you approach the patient who
has completed five years of an adjuvant aromatase inhibitor? DR LOVE: In the clinical setting, how do you approach the patient who
has completed five years of an adjuvant aromatase inhibitor? |
 DR SPARANO: I try to clarify for the patient which of the recommendations
that we are making are data driven, with unequivocal proof that this is the
appropriate choice, and which of our recommendations are not supported by
clear data but are based on our intuition, and then work with the patient to
devise a plan that best suits the situation.
DR SPARANO: I try to clarify for the patient which of the recommendations
that we are making are data driven, with unequivocal proof that this is the
appropriate choice, and which of our recommendations are not supported by
clear data but are based on our intuition, and then work with the patient to
devise a plan that best suits the situation.
For a patient who’s completed five years of up-front aromatase inhibitor
therapy, we don’t have the data, so my approach is to consider the patient’s risk
of recurrence as it was estimated at baseline and take into account how well
the patient tolerated the aromatase inhibitor, her age, comorbidities, et cetera.
 DR MACKEY: In the clinical setting, I believe the continuation of an aromatase
inhibitor beyond five years is purely speculative. We have a good grasp
regarding the toxicities with five years of an aromatase inhibitor — some of
the best data come from the ATAC trial — but some of the side effects could
be cumulative over time (ATAC Trialists’ Group 2006).
DR MACKEY: In the clinical setting, I believe the continuation of an aromatase
inhibitor beyond five years is purely speculative. We have a good grasp
regarding the toxicities with five years of an aromatase inhibitor — some of
the best data come from the ATAC trial — but some of the side effects could
be cumulative over time (ATAC Trialists’ Group 2006).
For example, we probably expect higher degrees of bone toxicity with
extended aromatase inhibitor therapy, but I believe one of the biggest worries
would be neuropsychiatric complications of long-term estrogen deprivation.
A lot of data from epidemiologic studies show that lower serum estrogens are
associated with a higher risk of dementia.
In my practice, I don’t continue patients beyond five years of an aromatase
inhibitor because of the lack of data and the potential for long-term effects.
 DR LOVE: Rowan, could you address this issue of cognitive functioning?
DR LOVE: Rowan, could you address this issue of cognitive functioning?
 DR CHLEBOWSKI: Evidence from a number of preclinical, observational
studies suggested that estrogen, especially exogenous estrogen, was associated
with favorable effects on cognition. Then the data from the Women’s
Health Initiative (WHI) randomized trial, with more than 16,000 otherwise
healthy postmenopausal women, demonstrated an increase in strokes associated
with estrogen use, and among women 65 years of age or older, dementia was
increased (Chlebowski 2006).
DR CHLEBOWSKI: Evidence from a number of preclinical, observational
studies suggested that estrogen, especially exogenous estrogen, was associated
with favorable effects on cognition. Then the data from the Women’s
Health Initiative (WHI) randomized trial, with more than 16,000 otherwise
healthy postmenopausal women, demonstrated an increase in strokes associated
with estrogen use, and among women 65 years of age or older, dementia was
increased (Chlebowski 2006).
Therefore, we have to worry that anything that increases arterial vascular
events will have an unfavorable effect on cognition, and in the ATAC trial, we see that anastrozole carries a significantly reduced risk of arterial vascular
effects compared to tamoxifen (ATAC Trialists’ Group 2006).
Thus, I’m less concerned about this issue. We don’t know what the effect of
aromatase inhibitors is on cognition, but I would need a new signal to become
concerned about that side effect with the long-term use of aromatase inhibitors.
 DR LOVE: MJ, one of the major options for patients completing five years
of an AI is participation in NSABP-B-42, which is evaluating an additional
five years of AI therapy. However, in a nonprotocol setting, how do you
approach women who are reaching five years on an aromatase inhibitor in
your practice?
DR LOVE: MJ, one of the major options for patients completing five years
of an AI is participation in NSABP-B-42, which is evaluating an additional
five years of AI therapy. However, in a nonprotocol setting, how do you
approach women who are reaching five years on an aromatase inhibitor in
your practice?
 DR JAHANZEB: I tell patients that emerging evidence shows that their risk of
recurrence persists and we don’t know whether it’s more beneficial to continue
or stop the aromatase inhibitor. Then, if they choose to continue, it’s informed
consent, and I find that approximately a third of my patients continue the
aromatase inhibitor, whereas the other two thirds don’t.
DR JAHANZEB: I tell patients that emerging evidence shows that their risk of
recurrence persists and we don’t know whether it’s more beneficial to continue
or stop the aromatase inhibitor. Then, if they choose to continue, it’s informed
consent, and I find that approximately a third of my patients continue the
aromatase inhibitor, whereas the other two thirds don’t.
Tracks 8-9
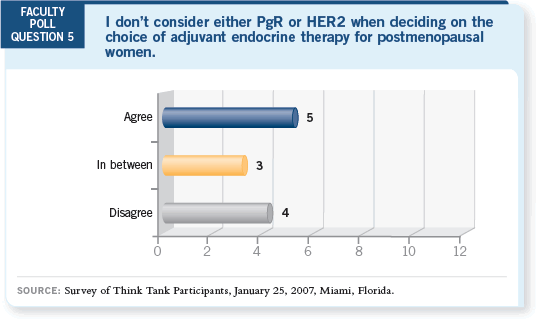
 DR LOVE: Mark, do you consider the tumor’s PR or HER2 status when
selecting adjuvant endocrine therapy? DR LOVE: Mark, do you consider the tumor’s PR or HER2 status when
selecting adjuvant endocrine therapy? |
 DR PEGRAM: At UCLA, we feel strongly that HER2 is a broad marker for
endocrine resistance independent of the type of antiestrogen therapy used, and
that’s what the data are bearing out, so I don’t see how that could be useful in decision-making. Also, the data indicate benefit from an aromatase inhibitor
in HER2-positive and in HER2-negative disease, and it appears that aromatase
inhibitors, as a class, are generally more active than tamoxifen regardless
of HER2 status.
DR PEGRAM: At UCLA, we feel strongly that HER2 is a broad marker for
endocrine resistance independent of the type of antiestrogen therapy used, and
that’s what the data are bearing out, so I don’t see how that could be useful in decision-making. Also, the data indicate benefit from an aromatase inhibitor
in HER2-positive and in HER2-negative disease, and it appears that aromatase
inhibitors, as a class, are generally more active than tamoxifen regardless
of HER2 status.
 DR GOSS: I believe the data are showing that although HER2 positivity is
a relative endocrine-resistance marker, both tamoxifen and aromatase inhibitors
are effective — it’s just that aromatase inhibitors, as in every other clinical
setting, are more effective. In my opinion, a higher relapse risk, such as a
HER2-positive or PR-negative tumor, more strongly justifies the use of an
up-front aromatase inhibitor over tamoxifen.
DR GOSS: I believe the data are showing that although HER2 positivity is
a relative endocrine-resistance marker, both tamoxifen and aromatase inhibitors
are effective — it’s just that aromatase inhibitors, as in every other clinical
setting, are more effective. In my opinion, a higher relapse risk, such as a
HER2-positive or PR-negative tumor, more strongly justifies the use of an
up-front aromatase inhibitor over tamoxifen.
 DR LOVE: Eric, how do you view the value of PR and HER2 in selecting an
adjuvant endocrine agent?
DR LOVE: Eric, how do you view the value of PR and HER2 in selecting an
adjuvant endocrine agent?
 DR WINER: I believe that both PR and HER2 are perhaps not predictive
factors but prognostic factors, and for a patient at higher risk of relapse in the
first few years, I am more inclined to use the aromatase inhibitor up front.
DR WINER: I believe that both PR and HER2 are perhaps not predictive
factors but prognostic factors, and for a patient at higher risk of relapse in the
first few years, I am more inclined to use the aromatase inhibitor up front.
 DR BUZDAR: If you examine BIG 1-98 or Dowsett’s data, on patients with
PR-negative and HER2-positive tumors, the benefit of aromatase inhibitors
over tamoxifen is modest, but it is still in the same direction (BIG 1-98
Collaborative Group 2005; Dowsett 2005).
DR BUZDAR: If you examine BIG 1-98 or Dowsett’s data, on patients with
PR-negative and HER2-positive tumors, the benefit of aromatase inhibitors
over tamoxifen is modest, but it is still in the same direction (BIG 1-98
Collaborative Group 2005; Dowsett 2005).
Select Publications

