
| Tracks 1-20 |
| Track 1 |
First-line therapy for patients with hormone receptor-positive, HER2-positive visceral metastases: Implications of the TAnDEM data |
| Track 2 |
Clinical implications of the
TAnDEM data for women with
asymptomatic metastatic disease |
| Track 3 |
Durable responses to a
combination of trastuzumab and
an aromatase inhibitor in the
palliative setting |
| Track 4 |
Incorporation of TCH and bevacizumab into the next generation of adjuvant trials for HER2-positive disease: Proposed NSABP/BCIRG adjuvant trial |
| Track 5 |
Adjuvant chemotherapeutic options to combine with trastuzumab |
| Track 6 |
Shifting treatment patterns in HER2-positive, early breast cancer |
| Track 7 |
Risk-benefit issues in the selection of adjuvant TCH for HER2-positive, early breast cancer |
| Track 8 |
Perspective on advances in the treatment of HER2-positive, early breast cancer |
| Track 9 |
Use of adjuvant trastuzumab for patients with smaller node-negative tumors |
|
| Track 10 |
Use of adjuvant trastuzumab monotherapy |
| Track 11 |
Optimal duration of adjuvant trastuzumab |
| Track 12 |
Reduced risk of cardiac toxicity with TCH |
| Track 13 |
Emerging adjuvant clinical trial strategies in HER2-positive disease |
| Track 14 |
The ALTTO trial: Trastuzumab,
lapatinib, the sequence or
combination with chemotherapy |
| Track 15 |
Cardiac safety issues in
combining trastuzumab and
bevacizumab in the adjuvant
setting |
| Track 16 |
Clinical equipoise in ALTTO and
the proposed NSABP/BCIRG
adjuvant trial in HER2-positive
disease |
| Track 17 |
Safety issues in trials of adjuvant bevacizumab |
| Track 18 |
Evaluation of cMYC as a prognostic factor in early breast cancer |
| Track 19 |
Evaluation of host and tumor factors in clinical trials |
| Track 20 |
Dose-dense AC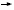 paclitaxel with trastuzumab for HER2-positive, early breast cancer paclitaxel with trastuzumab for HER2-positive, early breast cancer |
|
|
Select Excerpts from the Discussion
Tracks 1-2
 DR LOVE: John, how do you think through these three challenging
clinical cases, starting with the asymptomatic 60-year-old? DR LOVE: John, how do you think through these three challenging
clinical cases, starting with the asymptomatic 60-year-old? |
 DR MACKEY: I believe the standard approach is that with which the overall survival advantage has been demonstrated, which is with combination chemotherapy
and trastuzumab (Slamon 2001).
DR MACKEY: I believe the standard approach is that with which the overall survival advantage has been demonstrated, which is with combination chemotherapy
and trastuzumab (Slamon 2001).
The TAnDEM data didn’t change my approach for the average woman who comes in at age 60 with visceral metastases, particularly since we performed an
unplanned subgroup analysis of the women with liver metastases and they did
not appear to benefit from the addition of trastuzumab to anastrozole in terms
of overall survival. It was the women without liver metastases who might have
had a survival advantage associated with the addition of trastuzumab (Mackey
2006). If she were not willing to go through chemotherapy, I would talk to
her about the TAnDEM trial and trastuzumab with an aromatase inhibitor.
 DR GOSS: I want to remind people that in the population with HER2-negative disease, one of the most elegantly demonstrated points in the up-front
aromatase inhibitor trials was that visceral metastases respond extremely well.
I only make this point because I’m aware that in clinical practice, many people
still revert to chemotherapy when a patient has two or three asymptomatic
liver metastases.
DR GOSS: I want to remind people that in the population with HER2-negative disease, one of the most elegantly demonstrated points in the up-front
aromatase inhibitor trials was that visceral metastases respond extremely well.
I only make this point because I’m aware that in clinical practice, many people
still revert to chemotherapy when a patient has two or three asymptomatic
liver metastases.
 DR SPARANO: I agree with Paul entirely that endocrine therapy would be
the default position to take for patients with asymptomatic metastatic disease.
With regard to HER2-positive disease, I would favor saving trastuzumab for a
time when I needed to use chemotherapy because of the clear benefit when it
is added to chemotherapy in terms of objective response, time to progression
and overall survival.
DR SPARANO: I agree with Paul entirely that endocrine therapy would be
the default position to take for patients with asymptomatic metastatic disease.
With regard to HER2-positive disease, I would favor saving trastuzumab for a
time when I needed to use chemotherapy because of the clear benefit when it
is added to chemotherapy in terms of objective response, time to progression
and overall survival.
I would not use it when I’m administering endocrine therapy, when I believe
it brings less potential for benefit and I also have to tie the patient to receiving
parenteral therapy in addition to an oral therapy that’s well tolerated.
 DR PEGRAM: I believe that if you follow your patients closely, endocrine
therapy should be up for discussion with the patient, particularly in light of
its convenience. But we have to recognize from the TAnDEM trial that the
expectation from an aromatase inhibitor alone is modest, and the time of
benefit is short for the majority of patients (Mackey 2006).
DR PEGRAM: I believe that if you follow your patients closely, endocrine
therapy should be up for discussion with the patient, particularly in light of
its convenience. But we have to recognize from the TAnDEM trial that the
expectation from an aromatase inhibitor alone is modest, and the time of
benefit is short for the majority of patients (Mackey 2006).
As long as you capture those early progressions in a timely fashion and treat
them with trastuzumab-based therapy, which is arguably the most important
of the targeted therapies that you’re going to use for these patients, then that’s
fine. Close clinical follow-up is the key to the management of these cases if
you’re not going to start with a trastuzumab-based regimen.
Outside of that caveat, my preference would be to use a trastuzumab-based
regimen up front. I would probably start with an aromatase inhibitor in this
particular case because of the lack of side effects compared to cytotoxic therapy.
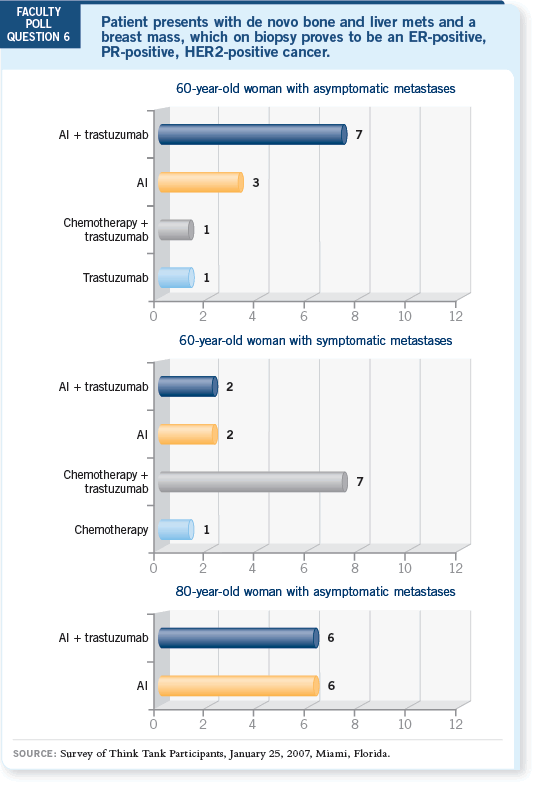
 DR DICKLER: I agree. In the TAnDEM trial, the median progression-free
survival went from 2.4 to 4.8 months with the addition of trastuzumab to
anastrozole (Mackey 2006; [1.1]). Some patients derive most of that benefit,
and others don’t derive any.
DR DICKLER: I agree. In the TAnDEM trial, the median progression-free
survival went from 2.4 to 4.8 months with the addition of trastuzumab to
anastrozole (Mackey 2006; [1.1]). Some patients derive most of that benefit,
and others don’t derive any.
Without being able to select those patients, I will probably use the combination
of trastuzumab and an aromatase inhibitor up front because some people
will derive a great benefit. Until I know who those patients are and I can select them when I’m starting therapy, I will administer the combination
initially.
I also feel that using hormonal therapy is important. I’ve had patients in my
practice with metastatic disease for eight to 10 years. It’s important to be able
to delay the onset of chemotherapy because it has a big impact on quality of
life.
 DR WINER: I didn’t find the TAnDEM trial to be practice changing to any
significant degree. Prior to TAnDEM, I would selectively use an aromatase
inhibitor for some patients and trastuzumab monotherapy for some patients.
Most patients with HER2-positive disease in my clinical practice receive some
form of chemotherapy with trastuzumab, and I will continue that practice.
DR WINER: I didn’t find the TAnDEM trial to be practice changing to any
significant degree. Prior to TAnDEM, I would selectively use an aromatase
inhibitor for some patients and trastuzumab monotherapy for some patients.
Most patients with HER2-positive disease in my clinical practice receive some
form of chemotherapy with trastuzumab, and I will continue that practice.
Track 5
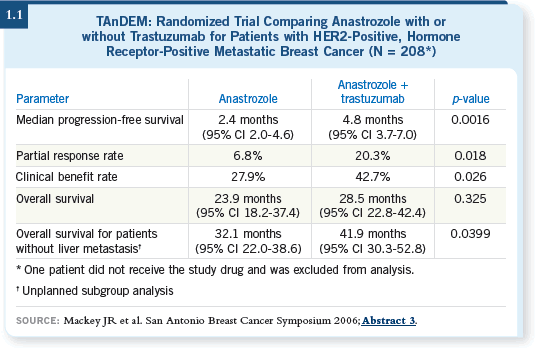
 DR LOVE: Let’s talk about adjuvant therapy of HER2-positive disease.
Eric, what chemotherapy regimen were you combining with trastuzumab
before the results of BCIRG 006 were reported at the 2006 San Antonio
Breast Cancer Symposium, and what are you using now? DR LOVE: Let’s talk about adjuvant therapy of HER2-positive disease.
Eric, what chemotherapy regimen were you combining with trastuzumab
before the results of BCIRG 006 were reported at the 2006 San Antonio
Breast Cancer Symposium, and what are you using now? |
 DR WINER: Prior to the San Antonio meeting, for most patients I used AC
followed by TH (paclitaxel/trastuzumab), the Intergroup regimen, and I
still do, although my comfort level has increased in terms of using TCH
(docetaxel/carboplatin/trastuzumab) for the patient who has me worried about
cardiac toxicity.
DR WINER: Prior to the San Antonio meeting, for most patients I used AC
followed by TH (paclitaxel/trastuzumab), the Intergroup regimen, and I
still do, although my comfort level has increased in terms of using TCH
(docetaxel/carboplatin/trastuzumab) for the patient who has me worried about
cardiac toxicity.
We have years and thousands of patients of experience using anthracyclines in
this setting of HER2-positive disease. I’m not ready to shift everyone, but it’s
interesting.
So my questions are: Do you believe carboplatin makes any difference? In an
ideal world, wouldn’t you like to suddenly find 10,000 patients to ask whether
you can use even kinder and gentler chemotherapy with trastuzumab?
My guess is that trastuzumab in the end is the great equalizer and that the
chemotherapy doesn’t make a difference as long as you use some.
 DR LOVE: Mark, do we need the carboplatin in TCH?
DR LOVE: Mark, do we need the carboplatin in TCH?
 DR PEGRAM: The docetaxel/trastuzumab (TH) versus TCH question has
been addressed in a randomized trial for patients with metastatic breast cancer.
It is not kinder and gentler because in that trial the dose of docetaxel was 100
mg/m2 in the TH arm and 75 mg/m2 in the TCH arm (Forbes 2006).
DR PEGRAM: The docetaxel/trastuzumab (TH) versus TCH question has
been addressed in a randomized trial for patients with metastatic breast cancer.
It is not kinder and gentler because in that trial the dose of docetaxel was 100
mg/m2 in the TH arm and 75 mg/m2 in the TCH arm (Forbes 2006).
The toxicity was arguably higher in the TH arm compared to the TCH arm,
and the efficacy was equivalent in both arms (Forbes 2006). In terms of the
therapeutic index, you could argue, based on that study, that TCH would still
come out the winner.
 DR LOVE: Mark, for a 62-year-old woman with HER2-positive disease with
two positive nodes who is otherwise healthy, what are you likely to recommend
as adjuvant therapy outside of a study?
DR LOVE: Mark, for a 62-year-old woman with HER2-positive disease with
two positive nodes who is otherwise healthy, what are you likely to recommend
as adjuvant therapy outside of a study?
 DR PEGRAM: I will definitely discuss both treatment options with the patient
— TCH or an anthracycline/taxane/trastuzumab regimen — and I would
point out the various toxicity profiles and ascertain the patient’s impression of
what she would like to choose. I would not say that the patient must receive
TCH, but I believe it’s reasonable to offer it as a treatment option.
DR PEGRAM: I will definitely discuss both treatment options with the patient
— TCH or an anthracycline/taxane/trastuzumab regimen — and I would
point out the various toxicity profiles and ascertain the patient’s impression of
what she would like to choose. I would not say that the patient must receive
TCH, but I believe it’s reasonable to offer it as a treatment option.
I agree that it’s reasonable to be conservative with regard to changing clinical
practice based on an abstract presentation of an interim analysis (Slamon
2006). As a general rule, those are rarely practice-changing presentations,
but this does bring up the issue of a nonanthracycline-based chemotherapy
regimen to integrate with trastuzumab in the adjuvant setting.
It puts the option on the table, and it’s a reasonable treatment option. I agree
with Eric that trastuzumab probably is the great equalizer and the chemotherapy
base will perhaps become a secondary issue.
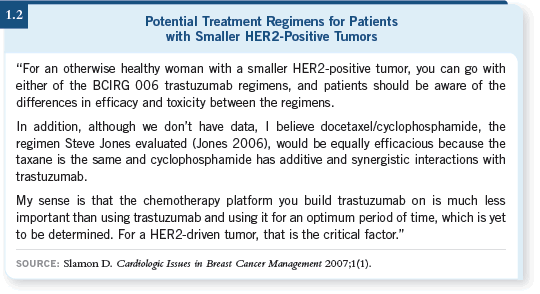
 DR LOVE: What about using TCH with the “C” being cyclophosphamide (1.2)?
DR LOVE: What about using TCH with the “C” being cyclophosphamide (1.2)?
 DR PEGRAM: The US Oncology network is exploring that combination
in ongoing trials. The hypothesis behind the synergy of trastuzumab and
cytotoxic agents extends equally to the alkylating agents, as it did for the
platinum salts. I believe that’s a reasonable substitution, and I will be interested to follow the data as they emerge.
DR PEGRAM: The US Oncology network is exploring that combination
in ongoing trials. The hypothesis behind the synergy of trastuzumab and
cytotoxic agents extends equally to the alkylating agents, as it did for the
platinum salts. I believe that’s a reasonable substitution, and I will be interested to follow the data as they emerge.
 DR LOVE: Matt, how are you approaching treatment for these patients right
now?
DR LOVE: Matt, how are you approaching treatment for these patients right
now?
 DR ELLIS: Before the BCIRG 006 data were reported at the 2006 San
Antonio meeting, I carefully evaluated the patient’s baseline ejection fraction
and age. This was because of the analysis conducted in NSABP-B-31 showing
that the older the patient and the lower the baseline ejection fraction, the more
likely a patient was to develop congestive heart failure with trastuzumab (Tan-
Chiu 2005).
DR ELLIS: Before the BCIRG 006 data were reported at the 2006 San
Antonio meeting, I carefully evaluated the patient’s baseline ejection fraction
and age. This was because of the analysis conducted in NSABP-B-31 showing
that the older the patient and the lower the baseline ejection fraction, the more
likely a patient was to develop congestive heart failure with trastuzumab (Tan-
Chiu 2005).
If the patient appeared to be in a higher-risk group — older or with a lower
baseline ejection fraction — I would use TCH. Now I’m even less likely to
use AC 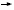 T, driven by the safety data.
T, driven by the safety data.
 DR LOVE: Again, a 62-year-old, healthy woman with a normal ejection
fraction: What are you likely to recommend?
DR LOVE: Again, a 62-year-old, healthy woman with a normal ejection
fraction: What are you likely to recommend?
 DR ELLIS: I agree with Mark. You have to work through the issues, and I’d
emphasize that I’d like to see five-year data. But at this point, I would tend
more towards TCH.
DR ELLIS: I agree with Mark. You have to work through the issues, and I’d
emphasize that I’d like to see five-year data. But at this point, I would tend
more towards TCH.
Track 7
 DR LOVE: Joe, what is your take on the BCIRG 006 presentation? DR LOVE: Joe, what is your take on the BCIRG 006 presentation? |
 DR SPARANO: BCIRG 006 was an important trial for two reasons. First,
when the data were initially presented, it was the third trial clearly showing a
dramatic improvement in outcome with adjuvant trastuzumab (Slamon 2005).
DR SPARANO: BCIRG 006 was an important trial for two reasons. First,
when the data were initially presented, it was the third trial clearly showing a
dramatic improvement in outcome with adjuvant trastuzumab (Slamon 2005).
Second, it put the TCH regimen on the table as an alternative, notwithstanding
the limitations due to the fact that the trial was not designed to
compare the two trastuzumab-containing regimens with regard to efficacy. It
was designed to compare the two experimental trastuzumab regimens to the
standard regimen without trastuzumab.
Nevertheless, it still puts that regimen on the table. Has it changed my
practice? Not necessarily. I still feel more comfortable with the totality of
evidence from all the studies using doxorubicin-based therapy.
Also, I find that when I meet with a patient, there’s so much information to
review, and she is often overwhelmed by all the components of therapy we
need to discuss. Therefore, I generally choose not to put this on the table
unless I believe it should be there because of my concerns related to cardiac
toxicity.
More relapses do appear to occur with TCH thus far. On the other hand, it
is associated with less cardiac toxicity. I’m not sure yet about leukemia — it’s
four cases for the anthracycline-based regimens versus zero cases for TCH. We need more time before we have an answer to that (Slamon 2006).
 DR LOVE: John, do you agree that more relapses occurred with TCH?
DR LOVE: John, do you agree that more relapses occurred with TCH?
 DR MACKEY: Statistically, no. The trial was designed — if both arms outperformed
the control — to provide a protocol-specified comparison of the
two arms. That protocol-specified comparison produces a p-value that is not
even close to a trend and, in fact, is heading in the direction of no difference
between the two arms (Slamon 2006). All I can say is that the efficacy
data indicate that they’re indistinguishable and the safety data, I believe, favor
TCH.
DR MACKEY: Statistically, no. The trial was designed — if both arms outperformed
the control — to provide a protocol-specified comparison of the
two arms. That protocol-specified comparison produces a p-value that is not
even close to a trend and, in fact, is heading in the direction of no difference
between the two arms (Slamon 2006). All I can say is that the efficacy
data indicate that they’re indistinguishable and the safety data, I believe, favor
TCH.
Tracks 9-10
 DR LOVE: Eric, can you comment on your decision-making process
for patients with ER-negative, HER2-positive tumors smaller than five
millimeters (T1a) and those with ER-negative, HER2-positive tumors
between five and 10 millimeters (T1b)? DR LOVE: Eric, can you comment on your decision-making process
for patients with ER-negative, HER2-positive tumors smaller than five
millimeters (T1a) and those with ER-negative, HER2-positive tumors
between five and 10 millimeters (T1b)? |
 DR WINER: The difference is pretty big in terms of the volume of those
tumors. I’ll draw a line in the sand at T1a. The woman with a T1b tumor I
will treat with trastuzumab and chemotherapy. I am not at the point where
I’m willing to do that for a patient with a T1aN0 tumor.
DR WINER: The difference is pretty big in terms of the volume of those
tumors. I’ll draw a line in the sand at T1a. The woman with a T1b tumor I
will treat with trastuzumab and chemotherapy. I am not at the point where
I’m willing to do that for a patient with a T1aN0 tumor.
 DR LOVE: What about an 8-mm tumor that is ER-positive and HER2-positive?
DR LOVE: What about an 8-mm tumor that is ER-positive and HER2-positive?
 DR WINER: I’m more on the fence.
DR WINER: I’m more on the fence.
 DR PEGRAM: The bottom line is that trastuzumab, not the endocrine therapy, will do the lion’s share of the work in terms of clinical benefit in these patients
with HER2-positive tumors. So trastuzumab must be considered the base
from which you formulate further combinations. If the patient had ER-positive
disease, it would be reasonable to add endocrine therapy.
DR PEGRAM: The bottom line is that trastuzumab, not the endocrine therapy, will do the lion’s share of the work in terms of clinical benefit in these patients
with HER2-positive tumors. So trastuzumab must be considered the base
from which you formulate further combinations. If the patient had ER-positive
disease, it would be reasonable to add endocrine therapy.
Although HER2 is a marker for relative endocrine resistance, it doesn’t mean
patients don’t obtain a benefit from endocrine therapy — they just derive less
benefit. So for a patient with a smaller ER-positive, HER2-positive tumor, I
believe it would be reasonable to consider an endocrine and HER2-directed
combination in the absence of chemotherapy.
 DR LOVE: Joe, in your practice, how do you approach patients with HER2-positive tumors that are smaller than one centimeter?
DR LOVE: Joe, in your practice, how do you approach patients with HER2-positive tumors that are smaller than one centimeter?
 DR SPARANO: If the patient has a HER2-positive tumor, she derives relatively
greater benefit from chemotherapy. Without question, she would also derive
benefit from trastuzumab. The critical question is, at what level of risk do you
pull the trigger? And when you pull the trigger, do you pull it once or twice?
DR SPARANO: If the patient has a HER2-positive tumor, she derives relatively
greater benefit from chemotherapy. Without question, she would also derive
benefit from trastuzumab. The critical question is, at what level of risk do you
pull the trigger? And when you pull the trigger, do you pull it once or twice?
If we pull the trigger, I feel obligated to use chemotherapy with trastuzumab. I
have used trastuzumab with endocrine therapy alone in certain circumstances,
and they tend to be older patients with high-risk disease, not patients with
smaller tumors.
 DR LOVE: Are you generally treating patients with a HER2-positive tumor
that is smaller than one centimeter?
DR LOVE: Are you generally treating patients with a HER2-positive tumor
that is smaller than one centimeter?
 DR SPARANO: Yes. Absolutely, because they do have a higher risk of recurrence,
as we know from the Oncotype DX™ recurrence score. I fall in Eric’s camp, and I draw a line in the sand for patients with tumors that are T1a or
less, at which I say, “We don’t need to use chemotherapy or trastuzumab in
that setting.”
DR SPARANO: Yes. Absolutely, because they do have a higher risk of recurrence,
as we know from the Oncotype DX™ recurrence score. I fall in Eric’s camp, and I draw a line in the sand for patients with tumors that are T1a or
less, at which I say, “We don’t need to use chemotherapy or trastuzumab in
that setting.”
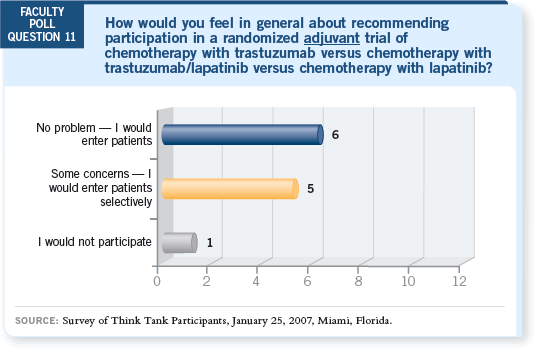
 DR LOVE: Kathy, how do you approach these patients?
DR LOVE: Kathy, how do you approach these patients?
 DR PRITCHARD: This decision is easy for me in Ontario because I am not
reimbursed for trastuzumab if the patient has a tumor that is smaller than one
centimeter. I also can’t use trastuzumab unless I also use chemotherapy.
DR PRITCHARD: This decision is easy for me in Ontario because I am not
reimbursed for trastuzumab if the patient has a tumor that is smaller than one
centimeter. I also can’t use trastuzumab unless I also use chemotherapy.
Left to my own devices, I would use trastuzumab for some patients with
tumors that are smaller than one centimeter, and I would use trastuzumab
alone for some patients who are elderly or frail.
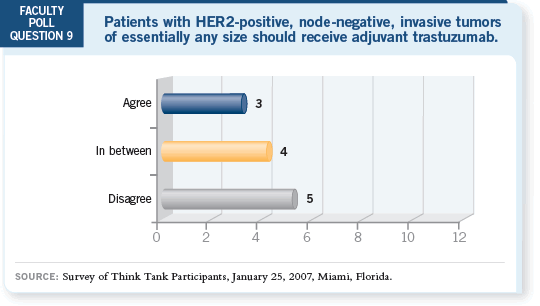
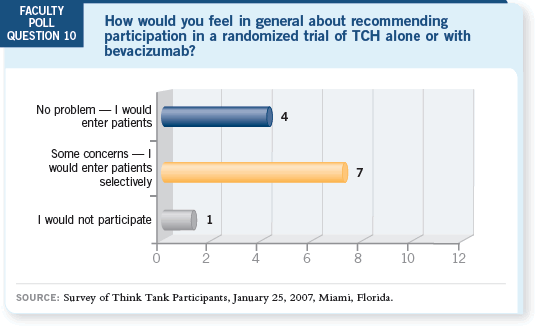
Tracks 13-14
 DR LOVE: What are your thoughts on the results from the poll questions
regarding participation in trials with lapatinib or bevacizumab for patients
with HER2-positive disease? DR LOVE: What are your thoughts on the results from the poll questions
regarding participation in trials with lapatinib or bevacizumab for patients
with HER2-positive disease? |
 DR MACKEY: I’m encouraged by this type of reaction because the BCIRG
006 data we’re talking about are six weeks old, and we are already shifting the
majority of the poll participants to considering these novel trial designs.
DR MACKEY: I’m encouraged by this type of reaction because the BCIRG
006 data we’re talking about are six weeks old, and we are already shifting the
majority of the poll participants to considering these novel trial designs.
We’ve struggled for 25 years to prove that anthracyclines matter. We finally
achieved a four percent disease-free survival advantage (EBCTCG 2005). We
have to admit that there may be more important issues to address, such as the biological questions, rather
than being stuck on what is an
old, toxic treatment.
 I believe we will see a great
deal of interest in these new
trials, in which the biology
drives the question (1.3).
Also, we will find increasingly
that we’re willing to
pursue regimens that require,
in a sense, a withdrawal of
some of the accepted past
treatments because we can’t
simply keep adding to the
toxicities by adding yet
another agent.
I believe we will see a great
deal of interest in these new
trials, in which the biology
drives the question (1.3).
Also, we will find increasingly
that we’re willing to
pursue regimens that require,
in a sense, a withdrawal of
some of the accepted past
treatments because we can’t
simply keep adding to the
toxicities by adding yet
another agent.
 DR PRITCHARD: It would be nice to see the TCH data published in a peer-reviewed
setting before we gallop off in any direction.
DR PRITCHARD: It would be nice to see the TCH data published in a peer-reviewed
setting before we gallop off in any direction.
Having said that, I believe logic and momentum are behind this as a concept.
That’s why you’re seeing people considering it fairly quickly. This would be a
reasonable trial with a reasonable control arm.
 DR LOVE: Would you be comfortable with the ALTTO (Adjuvant Lapatinib
and/or Trastuzumab Treatment Optimization) trial, which includes a treatment
arm without trastuzumab?
DR LOVE: Would you be comfortable with the ALTTO (Adjuvant Lapatinib
and/or Trastuzumab Treatment Optimization) trial, which includes a treatment
arm without trastuzumab?
 DR PRITCHARD: I’m comfortable with both trials.
DR PRITCHARD: I’m comfortable with both trials.
 DR LOVE: Aman, for a patient with multiple positive nodes, how comfortable
are you with the ALTTO trial, in which she might not receive trastuzumab?
DR LOVE: Aman, for a patient with multiple positive nodes, how comfortable
are you with the ALTTO trial, in which she might not receive trastuzumab?
 DR BUZDAR: If the trial had a lapatinib arm, I would not have any reservations
because I believe lapatinib, at least in the setting for which we have
information, shows substantial antitumor activity, even in patients with trastuzumab-resistant disease.
DR BUZDAR: If the trial had a lapatinib arm, I would not have any reservations
because I believe lapatinib, at least in the setting for which we have
information, shows substantial antitumor activity, even in patients with trastuzumab-resistant disease.
 DR GOSS: I’m extremely pleased that Aman gave that answer because it
reminds me of the ATAC trial, when it was designed and initiated. At that
time, no patient with metastatic disease had ever been treated with anastrozole
as first-line therapy.
DR GOSS: I’m extremely pleased that Aman gave that answer because it
reminds me of the ATAC trial, when it was designed and initiated. At that
time, no patient with metastatic disease had ever been treated with anastrozole
as first-line therapy.
Second, no human being had ever been treated with the combination of
anastrozole and tamoxifen. A 9,000-patient trial was initiated in 1997 in
which the standard practice was abandoned in favor of a novel therapy, based
on a class effect.
That’s what the ALTTO trial is doing with lapatinib, which is another anti-
HER2 therapy. I believe this is correct. If you try to reinvent the wheel for each and every scenario with novel agents in a class, you’re going to slow
down the rate of discovery. So I concur with Aman’s answer exactly.
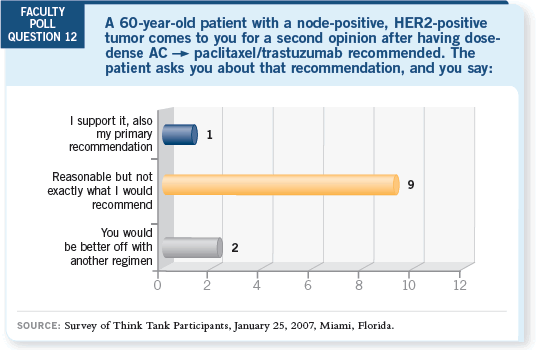
 DR LEYLAND-JONES: I have no problems with the ALTTO trial. In fact, I was
one of those who argued in favor of the lapatinib-alone arm.
DR LEYLAND-JONES: I have no problems with the ALTTO trial. In fact, I was
one of those who argued in favor of the lapatinib-alone arm.
The only concern I have with the trial of TCH with or without bevacizumab
is the fact that we currently have only 40 patients evaluated for cardiac risk
with the combination. So my concerns are the potential cardiac risk and the
fact that the database on the safety of the two drugs is so small.
Track 16
 DR WINER: Evidently we’ve all convinced ourselves that we’re okay with the
ALTTO trial. However, I don’t believe there’s a single person around the table
who wouldn’t like more data with lapatinib — something that would round
out what we know.
DR WINER: Evidently we’ve all convinced ourselves that we’re okay with the
ALTTO trial. However, I don’t believe there’s a single person around the table
who wouldn’t like more data with lapatinib — something that would round
out what we know.
I expect it’s likely we will have that over the course of the next six or 12
months. Of course, if we don’t have the data we need or if the data from the
ongoing studies cause concern, the ALTTO study will be adjusted as necessary.
The issues with the bevacizumab trial are somewhat different. I’m not bothered
by TCH being the control arm. I believe that’s an acceptable control. Given
concern about cardiac toxicity, it is, in fact, probably the best control arm.
If I were faced with a woman who had a small node-negative, HER2-positive tumor, I’m not sure I would want her to be randomly assigned to six cycles of
docetaxel/carboplatin and trastuzumab and bevacizumab. It’s potentially a lot
of therapy for that patient with relatively low-risk disease. In clinical trials, we
all make decisions about patients with which we have less equipoise.
Track 20
 DR LOVE: John, what are your thoughts about using dose-dense
AC DR LOVE: John, what are your thoughts about using dose-dense
AC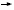 paclitaxel/ trastuzumab? paclitaxel/ trastuzumab? |
 DR MACKEY: When you have a number of good choices, why would you fly
by the seat of your pants with a regimen from a 70-patient study in a preselected
population? Because we have other good options, I wouldn’t go there.
I wouldn’t say that it’s wrong — it’s a reasonable recommendation — but it
wouldn’t be what I would recommend.
DR MACKEY: When you have a number of good choices, why would you fly
by the seat of your pants with a regimen from a 70-patient study in a preselected
population? Because we have other good options, I wouldn’t go there.
I wouldn’t say that it’s wrong — it’s a reasonable recommendation — but it
wouldn’t be what I would recommend.
 DR LOVE: Maura, can you comment on why so many people at Memorial
Sloan-Kettering are using this approach now?
DR LOVE: Maura, can you comment on why so many people at Memorial
Sloan-Kettering are using this approach now?
 DR DICKLER: The chemotherapy is well tolerated, and you move through it
quickly. I believe it is the ease of administration and the fact that dose-dense
AC
DR DICKLER: The chemotherapy is well tolerated, and you move through it
quickly. I believe it is the ease of administration and the fact that dose-dense
AC  T doesn’t increase cardiac events (Hudis 2005).
T doesn’t increase cardiac events (Hudis 2005).
Select Publications

