
| Tracks 1-10 |
| Track 1 |
Clinical implications of ECOG-E2100 and treatment with bevacizumab beyond the first-line setting |
| Track 2 |
Flexibility in expanding the scope of bevacizumab beyond use with paclitaxel in the first-line setting |
| Track 3 |
Side effects and tolerability of bevacizumab |
| Track 4 |
Long-term responders to capecitabine/bevacizumab in the metastatic setting |
| Track 5 |
Value of bevacizumab for patients
with asymptomatic metastatic
disease in the absence of a
survival advantage |
|
| Track 6 |
Expected benefit from the
incorporation of bevacizumab into
the adjuvant setting |
| Track 7 |
Advantage of shorter infusion
time and lack of premedication
with nanoparticle albumin-bound
(nab) paclitaxel |
| Track 8 |
Need for head-to-head clinical trials comparing nab paclitaxel with standard taxane dose and schedules |
| Track 9 |
Evaluation of nab paclitaxel in the adjuvant setting |
| Track 10 |
NSABP Phase II trial of neoadjuvant chemotherapy with sequential weekly nab paclitaxel followed by FEC |
|
|
Select Excerpts from the Discussion
Tracks 1-3, 6
 DR LOVE: Mark, can you comment on the use of bevacizumab for
patients with HER2-negative metastatic disease? DR LOVE: Mark, can you comment on the use of bevacizumab for
patients with HER2-negative metastatic disease? |
 DR PEGRAM: I was impressed by the ECOG-E2100 data (Miller 2005a). The
hazard ratio is reminiscent of our experience in the pivotal trial of trastuzumab
as first-line therapy for HER2-positive metastatic disease (Slamon
2001). Moreover, if you look at the one-year survival outcome differences,
you see that they also fall right on top of the one-year survival differences that
were recorded early on in the trastuzumab pivotal trial.
DR PEGRAM: I was impressed by the ECOG-E2100 data (Miller 2005a). The
hazard ratio is reminiscent of our experience in the pivotal trial of trastuzumab
as first-line therapy for HER2-positive metastatic disease (Slamon
2001). Moreover, if you look at the one-year survival outcome differences,
you see that they also fall right on top of the one-year survival differences that
were recorded early on in the trastuzumab pivotal trial.
Clearly we have demonstration of efficacy in terms of improved time to
tumor progression. The overall survival data are not yet mature for that data
set, but I would expect they should be this year. The last time Kathy Miller
updated that data set, I believe only about 30 percent of the final number of
survival events had occurred (Miller 2005a). So we’ll have to wait for the final
analysis.
 DR LOVE: What about the use of bevacizumab as second-line therapy?
DR LOVE: What about the use of bevacizumab as second-line therapy?
 DR PEGRAM: That question is being addressed in the ongoing RIBBON 2
study. Patients on that study are able to select from a menu of different chemotherapy
options at the investigator’s discretion, and then they are randomly
assigned to bevacizumab or placebo in the second-line setting. Short of any
data from such a trial, I probably would not routinely recommend bevacizumab
in a second-line setting because we have literally no data to support it
at this time.
DR PEGRAM: That question is being addressed in the ongoing RIBBON 2
study. Patients on that study are able to select from a menu of different chemotherapy
options at the investigator’s discretion, and then they are randomly
assigned to bevacizumab or placebo in the second-line setting. Short of any
data from such a trial, I probably would not routinely recommend bevacizumab
in a second-line setting because we have literally no data to support it
at this time.
 DR LEYLAND-JONES: In terms of activity in the second-line setting, Mark
is absolutely right. We have no data, but I have the feeling we will be seeing
significant activity with bevacizumab in the second- and third-line settings for
metastatic disease.
DR LEYLAND-JONES: In terms of activity in the second-line setting, Mark
is absolutely right. We have no data, but I have the feeling we will be seeing
significant activity with bevacizumab in the second- and third-line settings for
metastatic disease.
 DR WINER: I don’t believe you can think of this as another combination
therapy like a taxane with capecitabine or gemcitabine. It’s important to
recognize that a previous randomized trial of bevacizumab with capecitabine
in the second-line setting was largely negative, although a hint of activity was
evident (Miller 2005b).
DR WINER: I don’t believe you can think of this as another combination
therapy like a taxane with capecitabine or gemcitabine. It’s important to
recognize that a previous randomized trial of bevacizumab with capecitabine
in the second-line setting was largely negative, although a hint of activity was
evident (Miller 2005b).
I don’t believe we should be too rigid about defining first- and second-line
therapy because so much of this depends on what someone has received in the
adjuvant setting. A woman who received adjuvant TAC or AC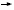 paclitaxel
nine months ago and now has a relapse is technically in the first-line setting
and far more refractory than many who are in the second-line setting who
might not have received adjuvant chemotherapy.
paclitaxel
nine months ago and now has a relapse is technically in the first-line setting
and far more refractory than many who are in the second-line setting who
might not have received adjuvant chemotherapy.
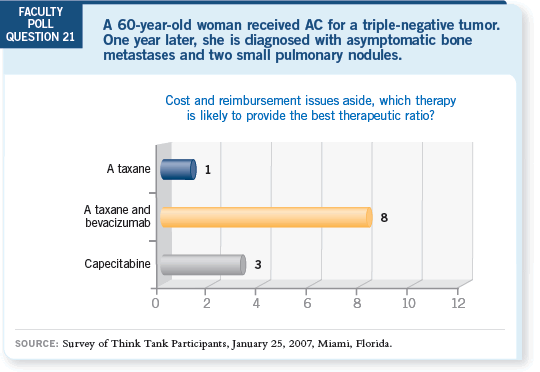
So for the woman who received adjuvant AC a few years ago and capecitabine
as her first-line regimen, I’m willing to try paclitaxel and bevacizumab, recognizing
that ECOG-E2100 limited eligibility to patients who had not received
chemotherapy in the metastatic setting.
 DR LOVE: Aman, what are your thoughts on this controversy?
DR LOVE: Aman, what are your thoughts on this controversy?
 DR BUZDAR: I don’t believe there is much controversy. The data clearly
demonstrated that inclusion of a biologic with paclitaxel substantially improved
the response rate and time to progression (Miller 2005a). This is a viable
positive lead, and we need to discuss it with every patient who meets those
eligibility criteria.
DR BUZDAR: I don’t believe there is much controversy. The data clearly
demonstrated that inclusion of a biologic with paclitaxel substantially improved
the response rate and time to progression (Miller 2005a). This is a viable
positive lead, and we need to discuss it with every patient who meets those
eligibility criteria.
The next generation of trials will answer more clearly whether in the second-and
third-line settings the inclusion of bevacizumab will enhance the response
rate.
 DR LOVE: Would you consider combination chemotherapy (a taxane and
capecitabine) and bevacizumab for a patient with rapidly progressive visceral
disease?
DR LOVE: Would you consider combination chemotherapy (a taxane and
capecitabine) and bevacizumab for a patient with rapidly progressive visceral
disease?
 DR BUZDAR: I would not because the safety data are not available. We don’t
know which dose of each drug we should use for that combination. That is
not an appropriate recommendation outside of the context of a clinical trial.
DR BUZDAR: I would not because the safety data are not available. We don’t
know which dose of each drug we should use for that combination. That is
not an appropriate recommendation outside of the context of a clinical trial.
 DR LOVE: Matt, what are your thoughts?
DR LOVE: Matt, what are your thoughts?
 DR ELLIS: If ECOG-E2100 does not show an overall survival advantage, then bevacizumab is another palliative drug for the treatment of metastatic
breast cancer with the potential to relieve symptoms. If that were the case, I
would not use bevacizumab for asymptomatic patients because they have no
symptoms to palliate. I would probably reserve it for patients who are heavily
symptomatic with visceral crisis, for whom response is critical. If bevacizumab
improves survival, my view will change.
DR ELLIS: If ECOG-E2100 does not show an overall survival advantage, then bevacizumab is another palliative drug for the treatment of metastatic
breast cancer with the potential to relieve symptoms. If that were the case, I
would not use bevacizumab for asymptomatic patients because they have no
symptoms to palliate. I would probably reserve it for patients who are heavily
symptomatic with visceral crisis, for whom response is critical. If bevacizumab
improves survival, my view will change.
 DR BUZDAR: Let’s say that no survival advantage is demonstrated. Still, in
metastatic disease, the idea is to control the disease for as long as possible. If
you start with a combination therapy of biologics and chemotherapy and you
control the disease in a much higher number of patients for a much longer
period, that is a major achievement from the patient perspective, even though
long-term survival may not be affected.
DR BUZDAR: Let’s say that no survival advantage is demonstrated. Still, in
metastatic disease, the idea is to control the disease for as long as possible. If
you start with a combination therapy of biologics and chemotherapy and you
control the disease in a much higher number of patients for a much longer
period, that is a major achievement from the patient perspective, even though
long-term survival may not be affected.
 DR DICKLER: We have a lot to learn about bevacizumab. As we learn more
about how it works and how to appropriately select patients, then we’ll better
know with whom we should use it.
DR DICKLER: We have a lot to learn about bevacizumab. As we learn more
about how it works and how to appropriately select patients, then we’ll better
know with whom we should use it.
Right now, however, I believe stringent rules about which line of therapy to
use it in don’t make a lot of sense, and toxicity is an issue that must be considered
with this drug. Side effects include hypertension, which can be significant. Some patients require two drugs to control their blood pressure, but it is
controllable. I’ve also had patients develop nephrotic-range proteinuria, and I
monitor the urine protein-to-creatinine ratio every few months.
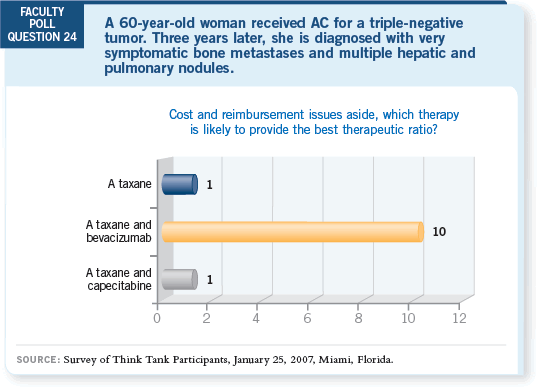
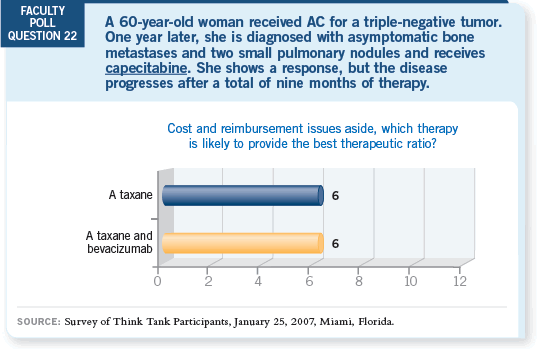
 DR LOVE: Joe, if a patient who received adjuvant AC develops asymptomatic
metastatic disease that is treated with capecitabine and then develops symptoms
from her metastatic disease, what would you recommend?
DR LOVE: Joe, if a patient who received adjuvant AC develops asymptomatic
metastatic disease that is treated with capecitabine and then develops symptoms
from her metastatic disease, what would you recommend?
 DR SPARANO: I would absolutely offer that patient paclitaxel and bevacizumab.
DR SPARANO: I would absolutely offer that patient paclitaxel and bevacizumab.
 DR ELLIS: So would I, probably.
DR ELLIS: So would I, probably.
 DR WINER: As would I.
DR WINER: As would I.
Tracks 7-8
 DR LOVE: MJ, let’s talk about the most recently available taxane, nab paclitaxel. Can you comment on the shorter infusion time for nab? DR LOVE: MJ, let’s talk about the most recently available taxane, nab paclitaxel. Can you comment on the shorter infusion time for nab? |
 DR JAHANZEB: From the practice standpoint, chair time is an issue, so this is
an advantage. Patients don’t want to sit for an infusion any longer than necessary.
Not having to administer premedications is another advantage with nab paclitaxel.
DR JAHANZEB: From the practice standpoint, chair time is an issue, so this is
an advantage. Patients don’t want to sit for an infusion any longer than necessary.
Not having to administer premedications is another advantage with nab paclitaxel.
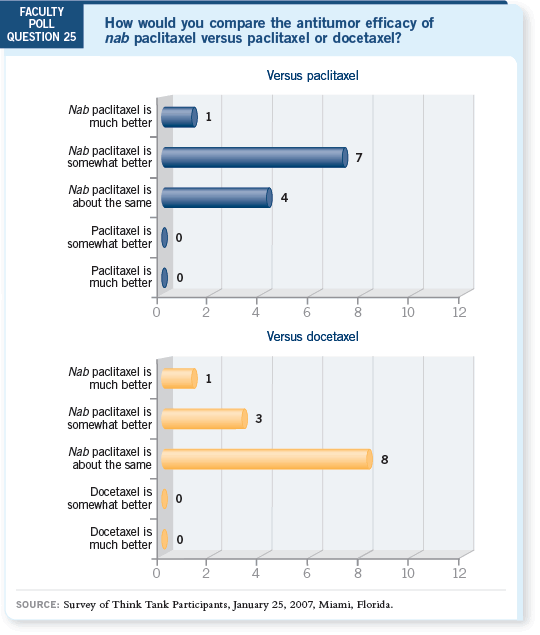
Also, the lack of allergic reactions and the shorter duration of neuropathy are
desirable (Gradishar 2005).
Cost is the only remaining issue with nab paclitaxel from a practitioner’s standpoint.
That has been the reason, I believe, for slower uptake. Otherwise, it’s a
good advance in terms of making a widely used drug better with respect to its
efficacy and toxicity.
 DR LOVE: Let me quickly poll the group. If the cost of nab paclitaxel were
exactly the same as paclitaxel, would you use paclitaxel? Show of hands is
unanimous with one exception. Eric, you are the only one not raising a hand.
DR LOVE: Let me quickly poll the group. If the cost of nab paclitaxel were
exactly the same as paclitaxel, would you use paclitaxel? Show of hands is
unanimous with one exception. Eric, you are the only one not raising a hand.
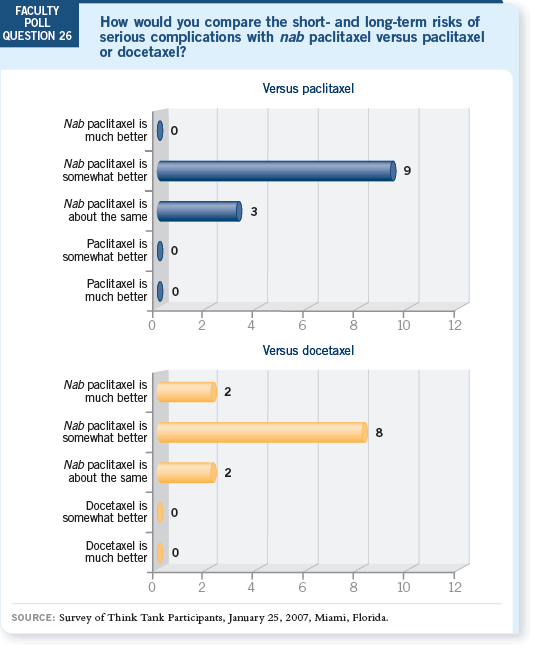
 DR WINER: Show me the Phase III trial that has compared nab paclitaxel to
weekly paclitaxel. I believe the claim that the neuropathy is of shorter duration
is based on a very small number of patients. I’m not aware of any symptom
complex that is typically more severe but goes away more quickly with one
drug versus another.
DR WINER: Show me the Phase III trial that has compared nab paclitaxel to
weekly paclitaxel. I believe the claim that the neuropathy is of shorter duration
is based on a very small number of patients. I’m not aware of any symptom
complex that is typically more severe but goes away more quickly with one
drug versus another.
 DR LOVE: From a quality-of-life point of view, Eric, how much of an advantage
are the shorter infusion time and lack of premedications?
DR LOVE: From a quality-of-life point of view, Eric, how much of an advantage
are the shorter infusion time and lack of premedications?
 DR WINER: If you’re talking about weekly versus weekly, weekly paclitaxel is administered over an hour, which is not a tremendously long time. The
need for ongoing steroid premedication when you’re using weekly paclitaxel is
something that one can question. Maybe nab paclitaxel will be a better drug,
but it’s important to investigate further.
DR WINER: If you’re talking about weekly versus weekly, weekly paclitaxel is administered over an hour, which is not a tremendously long time. The
need for ongoing steroid premedication when you’re using weekly paclitaxel is
something that one can question. Maybe nab paclitaxel will be a better drug,
but it’s important to investigate further.
 DR LOVE: Rowan?
DR LOVE: Rowan?
 DR CHLEBOWSKI: Up to now, docetaxel at 100 mg/m2 every three weeks
hasn’t been beaten by anything in the metastatic disease setting, but it has been
tied by weekly paclitaxel.
DR CHLEBOWSKI: Up to now, docetaxel at 100 mg/m2 every three weeks
hasn’t been beaten by anything in the metastatic disease setting, but it has been
tied by weekly paclitaxel.
The presentation by Gradishar at the 2006 San Antonio Breast Cancer Symposium
was a Phase II trial, but an apparently substantial improvement occurred
in the primary study endpoint, which was objective response, with weekly nab paclitaxel compared to every three-week docetaxel (Gradishar 2006). This is
impressive.
 DR LOVE: Maura, can you discuss your research experience with dose-dense
AC
DR LOVE: Maura, can you discuss your research experience with dose-dense
AC nab paclitaxel?
nab paclitaxel?
 DR DICKLER: We have a feasibility study (MSKCC-06019) evaluating bevacizumab
in the adjuvant setting, which is using dose-dense AC
DR DICKLER: We have a feasibility study (MSKCC-06019) evaluating bevacizumab
in the adjuvant setting, which is using dose-dense AC dose-dense
nab paclitaxel. The trial is currently accruing. Approximately 45 people have
enrolled, and many haven’t finished receiving the nab paclitaxel. I hope we’ll
have more information by ASCO.
dose-dense
nab paclitaxel. The trial is currently accruing. Approximately 45 people have
enrolled, and many haven’t finished receiving the nab paclitaxel. I hope we’ll
have more information by ASCO.
 DR LOVE: The US Oncology trial indicated that you need growth factors in
that situation.
DR LOVE: The US Oncology trial indicated that you need growth factors in
that situation.
 DR DICKLER: Correct. When they conducted their small pilot trial, they
didn’t use pegfilgrastim with nab paclitaxel at 260 mg/m2 every two weeks. I
believe a third of the patients couldn’t receive nab paclitaxel on time (Robert
2005). We’ve also conducted a study at Memorial Sloan-Kettering in which
we tried to avoid pegfilgrastim with dose-dense treatment, and we could not
administer the treatment on time. It required delays.
DR DICKLER: Correct. When they conducted their small pilot trial, they
didn’t use pegfilgrastim with nab paclitaxel at 260 mg/m2 every two weeks. I
believe a third of the patients couldn’t receive nab paclitaxel on time (Robert
2005). We’ve also conducted a study at Memorial Sloan-Kettering in which
we tried to avoid pegfilgrastim with dose-dense treatment, and we could not
administer the treatment on time. It required delays.
Select Publications

