
| Tracks 1-22 |
| Track 1 |
Dose-dense AC paclitaxel with trastuzumab for HER2-positive, early breast cancer (continued) paclitaxel with trastuzumab for HER2-positive, early breast cancer (continued) |
| Track 2 |
Benefits of adjuvant
chemotherapy for patients with
hormone receptor-positive
disease |
| Track 3 |
Selection of adjuvant
chemotherapy for patients
with node-positive breast
cancer |
| Track 4 |
Adjuvant dose-dense AC without a taxane |
| Track 5 |
Antitumor efficacy of dose-dense AC |
| Track 6 |
Taxane scheduling in adjuvant chemotherapy |
| Track 7 |
Clinical use of dose-dense AC paclitaxel/trastuzumab paclitaxel/trastuzumab |
| Track 8 |
Correlative tissue studies to identify molecular predictors of response to therapies |
| Track 9 |
Long-term implications of trastuzumab-
associated cardiotoxicity |
| Track 10 |
A devolving role for adjuvant chemotherapy in select patient subsets |
| Track 11 |
US Oncology trial of adjuvant docetaxel/cyclophosphamide (TC) versus AC |
|
| Track 12 |
Tolerability and side effects of adjuvant TC |
| Track 13 |
Adjuvant TC for patients with lower-risk, early breast cancer |
| Track 14 |
Capecitabine as a potential alternative to CMF in the adjuvant setting |
| Track 15 |
Implications of ECOG-E2197
comparing adjuvant doxorubicin/
docetaxel to AC chemotherapy |
| Track 16 |
Concerns about long-term anthracycline-associated cardiotoxicity |
| Track 17 |
TAILORx: Hormone therapy with or without chemotherapy for patients with intermediate recurrence scores on Oncotype DX |
| Track 18 |
Defining intermediate recurrence scores in TAILORx |
| Track 19 |
Use of standard prognostic factors to estimate recurrence risk |
| Track 20 |
Poor reliability and precision in standard clinical assays |
| Track 21 |
Advantages of the Oncotype DX assay’s precision and ability to identify predictive factors |
| Track 22 |
Use of the Oncotype DX multigene assay in clinical practice |
|
|
Select Excerpts from the Discussion
Tracks 1, 3-5
 DR LOVE: Can you comment on the adjuvant chemotherapy regimen you
use for patients with node-positive, HER2-negative disease? DR LOVE: Can you comment on the adjuvant chemotherapy regimen you
use for patients with node-positive, HER2-negative disease? |
 DR MACKEY: At our center, we would use TAC. Dose-dense AC
DR MACKEY: At our center, we would use TAC. Dose-dense AC paclitaxel
is also a good and reasonable option.
paclitaxel
is also a good and reasonable option.
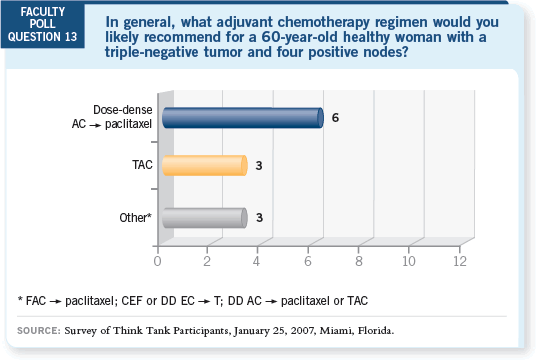
 DR JAHANZEB: We use TAC most often at our institution. The use of dose-dense
chemotherapy is notably regional. We are far enough away from New
York that we don’t use it.
DR JAHANZEB: We use TAC most often at our institution. The use of dose-dense
chemotherapy is notably regional. We are far enough away from New
York that we don’t use it.
 DR DICKLER: Do you use prophylactic antibiotics and growth factors?
DR DICKLER: Do you use prophylactic antibiotics and growth factors?
 DR JAHANZEB: Yes, as a rule, because a febrile neutropenia rate of 24 percent
is well above the NCCN guideline’s threshold above which they would be
routinely used.
DR JAHANZEB: Yes, as a rule, because a febrile neutropenia rate of 24 percent
is well above the NCCN guideline’s threshold above which they would be
routinely used.
 DR LOVE: Joe, you’re in the New York area. What do you do?
DR LOVE: Joe, you’re in the New York area. What do you do?
 DR SPARANO: My preference for the dose-dense regimen is due to the fact
that even if it’s not more effective than using the same drugs every three
weeks, it is completed in one third of the time. It is also associated with less
toxicity. I, and many people, have concerns about the toxicity associated with
TAC.
DR SPARANO: My preference for the dose-dense regimen is due to the fact
that even if it’s not more effective than using the same drugs every three
weeks, it is completed in one third of the time. It is also associated with less
toxicity. I, and many people, have concerns about the toxicity associated with
TAC.
 DR LOVE: What are your thoughts about using dose-dense AC without a
taxane?
DR LOVE: What are your thoughts about using dose-dense AC without a
taxane?
 DR SPARANO: I was the one person who said, “Yes, commonly.” The reason
for that is twofold. First, I was strongly reassured by the long-term safety data
with dose-dense therapy from CALGB-9741 with regard to cardiac toxicity
and secondary leukemia (Hudis 2005). Second, the Canadian trial (MA21)
demonstrated that some treatment effect might be achieved by shortening the
interval for only the anthracycline component of therapy (Burnell 2006).
DR SPARANO: I was the one person who said, “Yes, commonly.” The reason
for that is twofold. First, I was strongly reassured by the long-term safety data
with dose-dense therapy from CALGB-9741 with regard to cardiac toxicity
and secondary leukemia (Hudis 2005). Second, the Canadian trial (MA21)
demonstrated that some treatment effect might be achieved by shortening the
interval for only the anthracycline component of therapy (Burnell 2006).
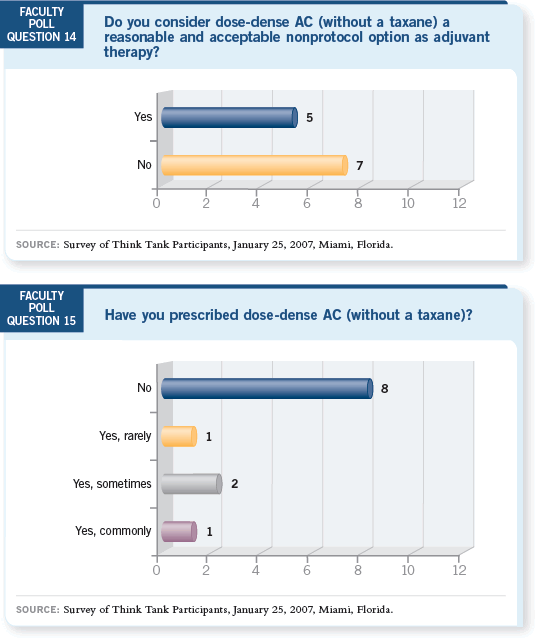
 DR WINER: I said, “Yes, sometimes.” I would be a “No” if it weren’t for the
fact that we have an ongoing CALGB study of dose-dense AC and colleagues
at my institution occasionally use this regimen.
DR WINER: I said, “Yes, sometimes.” I would be a “No” if it weren’t for the
fact that we have an ongoing CALGB study of dose-dense AC and colleagues
at my institution occasionally use this regimen.
I take the position that if you’re going to use AC, use it the way it’s been used
before. I worry that there might be a duration of therapy that’s too short and
that six weeks from beginning to end may be a problem. That said, I can’t
jump up and down and say it’s a crazy thing to do.
Tracks 11-15
 DR LOVE: Rowan, are you using the docetaxel/cyclophosphamide (TC)
regimen in your clinical practice? DR LOVE: Rowan, are you using the docetaxel/cyclophosphamide (TC)
regimen in your clinical practice? |
 DR CHLEBOWSKI: Yes. I like this combination, and we’ve adopted it as our
low-risk regimen. The TC regimen is easy to administer, and patients don’t
experience nausea and vomiting as they do with AC. With regard to long-term
risk, I would be willing to swap docetaxel for an anthracycline. Although
we have only one trial, it demonstrated a nice difference (Jones 2005).
DR CHLEBOWSKI: Yes. I like this combination, and we’ve adopted it as our
low-risk regimen. The TC regimen is easy to administer, and patients don’t
experience nausea and vomiting as they do with AC. With regard to long-term
risk, I would be willing to swap docetaxel for an anthracycline. Although
we have only one trial, it demonstrated a nice difference (Jones 2005).
 DR LOVE: Joe, how do you feel about the data Jones reported?
DR LOVE: Joe, how do you feel about the data Jones reported?
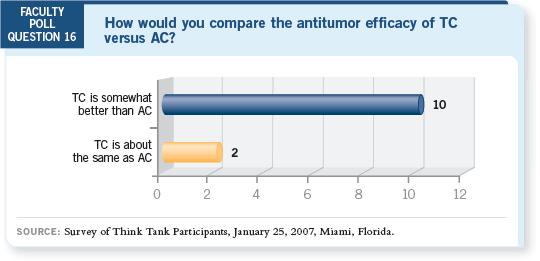
 DR SPARANO: This is an important study. It’s clearly positive and took many
of us by surprise. It goes against our biases as medical oncologists in terms of
our belief that doxorubicin is an effective drug and that, rather than replacing
it, we should substitute the cyclophosphamide with docetaxel. That’s what we
did in ECOG trial E2197, which compared doxorubicin/docetaxel to doxorubicin/
cyclophosphamide, and that resulted in a negative study (Goldstein
2005).
DR SPARANO: This is an important study. It’s clearly positive and took many
of us by surprise. It goes against our biases as medical oncologists in terms of
our belief that doxorubicin is an effective drug and that, rather than replacing
it, we should substitute the cyclophosphamide with docetaxel. That’s what we
did in ECOG trial E2197, which compared doxorubicin/docetaxel to doxorubicin/
cyclophosphamide, and that resulted in a negative study (Goldstein
2005).
What’s nice about the Jones data is that the treatment effect is nearly identical
to that seen in the paclitaxel arm of CALGB-9344, the Phase III study of
adjuvant cyclophosphamide/doxorubicin with or without paclitaxel, but
without the duration issue (Henderson 2003). It also removes from the
regimen a drug — doxorubicin — that we’re all concerned about with regard
to long-term toxicity.
 DR LOVE: What about tolerance of AC versus TC?
DR LOVE: What about tolerance of AC versus TC?
 DR SPARANO: My experience is anecdotal, but the data speak for themselves.
It is a tradeoff in terms of certain toxicities. One of the problems is that when we consider docetaxel, we generally think of TAC, which is clearly more toxic
than some of the other regimens. I believe that as clinicians use TC, they’ll
become more comfortable with it and we will see greater use of it.
DR SPARANO: My experience is anecdotal, but the data speak for themselves.
It is a tradeoff in terms of certain toxicities. One of the problems is that when we consider docetaxel, we generally think of TAC, which is clearly more toxic
than some of the other regimens. I believe that as clinicians use TC, they’ll
become more comfortable with it and we will see greater use of it.
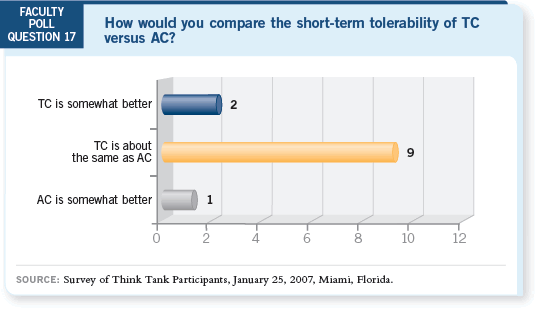
 DR PEGRAM: I’ve used TC a lot since the data were first presented (Jones
2005). It is our standard treatment for patients at high risk with node-negative
disease for whom we are not considering longer-duration or more intense
combinations.
DR PEGRAM: I’ve used TC a lot since the data were first presented (Jones
2005). It is our standard treatment for patients at high risk with node-negative
disease for whom we are not considering longer-duration or more intense
combinations.
A certain tradeoff is evident in terms of neutropenia, but it’s relatively low risk
and manageable. Clearly the TC regimen has less potential for upper gastrointestinal
toxicity, and it avoids the issue of cardiotoxicity altogether.
 DR GOSS: Does anyone here use four cycles of AC anymore?
DR GOSS: Does anyone here use four cycles of AC anymore?
 DR PEGRAM: Not alone.
DR PEGRAM: Not alone.
 DR PRITCHARD: Does anyone still use CMF? It’s definitely not cardiotoxic, it
doesn’t cause leukemia and it’s as effective as AC.
DR PRITCHARD: Does anyone still use CMF? It’s definitely not cardiotoxic, it
doesn’t cause leukemia and it’s as effective as AC.
 DR PEGRAM: Yes.
DR PEGRAM: Yes.
 DR ELLIS: I would like to comment on that. What the TC data have done
for me is put to rest my 10-year struggle with CMF. I agree with Kathy that
CMF has been a reasonable regimen to consider for many patients, particularly
patients with ER-positive, HER2-negative disease.
DR ELLIS: I would like to comment on that. What the TC data have done
for me is put to rest my 10-year struggle with CMF. I agree with Kathy that
CMF has been a reasonable regimen to consider for many patients, particularly
patients with ER-positive, HER2-negative disease.
The conundrum with CMF has always been oral versus IV cyclophosphamide.
In the classic NSABP-B-15 comparison of AC to CMF, oral cyclophosphamide
was used, and that is a difficult regimen to administer (Fisher 1990).
Now I can administer TC and forget CMF, which for me is a huge relief. I’m no longer torturing myself over administering IV CMF and then worrying
that I’m undertreating patients.
 DR DICKLER: Although alopecia does occur with the TC regimen, and
women hate to lose their hair.
DR DICKLER: Although alopecia does occur with the TC regimen, and
women hate to lose their hair.
 DR PEGRAM: Yes, but it’s only 12 weeks versus six months of therapy.
DR PEGRAM: Yes, but it’s only 12 weeks versus six months of therapy.
 DR ELLIS: If you discuss it with your patients, I believe most women will
trade some hair loss for finishing up treatment more quickly.
DR ELLIS: If you discuss it with your patients, I believe most women will
trade some hair loss for finishing up treatment more quickly.
 DR CHLEBOWSKI: Another option is capecitabine. At the San Antonio
meeting, data were presented from a randomized, 300-patient comparison of
two schedules of capecitabine versus CMF in advanced disease, and a survival
advantage and less toxicity were seen with capecitabine (Stockler 2006). I’m
not using CMF in the adjuvant setting, but given these data, I wonder why
anybody would want to use CMF in any breast cancer treatment setting.
DR CHLEBOWSKI: Another option is capecitabine. At the San Antonio
meeting, data were presented from a randomized, 300-patient comparison of
two schedules of capecitabine versus CMF in advanced disease, and a survival
advantage and less toxicity were seen with capecitabine (Stockler 2006). I’m
not using CMF in the adjuvant setting, but given these data, I wonder why
anybody would want to use CMF in any breast cancer treatment setting.
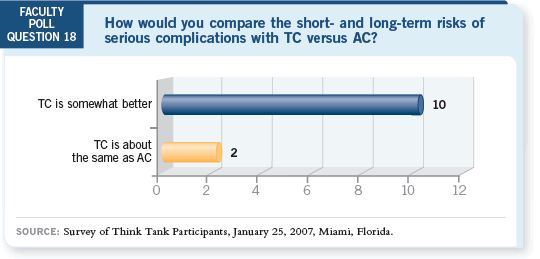
 DR WINER: I don’t have trouble administering TC, and I agree that it’s
probably less toxic. However, doesn’t it bother anyone that the well-executed
Intergroup trial, ECOG-E2197, which compared doxorubicin/docetaxel to
doxorubicin/cyclophosphamide, showed no benefit and yet the Jones data
suggest that docetaxel is better (Goldstein 2005; Jones 2005)?
DR WINER: I don’t have trouble administering TC, and I agree that it’s
probably less toxic. However, doesn’t it bother anyone that the well-executed
Intergroup trial, ECOG-E2197, which compared doxorubicin/docetaxel to
doxorubicin/cyclophosphamide, showed no benefit and yet the Jones data
suggest that docetaxel is better (Goldstein 2005; Jones 2005)?
If anything, I would have expected that substituting docetaxel for cyclophosphamide
would provide a bigger hit. I find it troublesome.
 DR LOVE: Do you have any explanation?
DR LOVE: Do you have any explanation?
 DR WINER: I don’t have an explanation, and it’s why, based on this one study,
I would conclude that TC is about the same as AC. I’m not ready to say it’s
better based on one study of 1,000 patients, given that the other study has a
result that causes concern.
DR WINER: I don’t have an explanation, and it’s why, based on this one study,
I would conclude that TC is about the same as AC. I’m not ready to say it’s
better based on one study of 1,000 patients, given that the other study has a
result that causes concern.
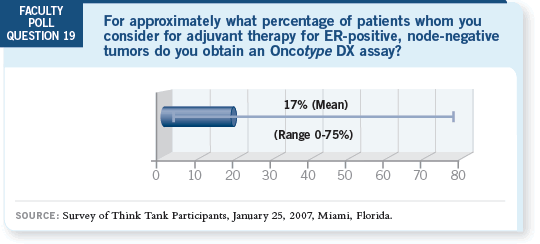
 DR LOVE: Joe, how many patients were in the E2197 trial?
DR LOVE: Joe, how many patients were in the E2197 trial?
 DR SPARANO: Approximately 2,900 patients were enrolled, and although the
four-year disease-free survival was projected to be about 78 percent in the AC
arm, it was actually 87 percent in both arms (Goldstein 2005).
DR SPARANO: Approximately 2,900 patients were enrolled, and although the
four-year disease-free survival was projected to be about 78 percent in the AC
arm, it was actually 87 percent in both arms (Goldstein 2005).
 DR BUZDAR: I agree with Eric. The E2197 trial did not show any subset of
patients who derived an advantage, or a hint of an advantage, when docetaxel
replaced cyclophosphamide.
DR BUZDAR: I agree with Eric. The E2197 trial did not show any subset of
patients who derived an advantage, or a hint of an advantage, when docetaxel
replaced cyclophosphamide.
A consistent, slightly inferior outcome was evident from combining docetaxel
with an anthracycline, so I would be cautious when interpreting the study
comparing AC to TC with a smaller sample size. I believe it should be
confirmed before we jump on the bandwagon.
 DR WINER: Don’t get me wrong. I’m pondering whether I should go back to
my practice and consider using TC more often — I’m just not ready to tell
patients that I know it’s a better regimen.
DR WINER: Don’t get me wrong. I’m pondering whether I should go back to
my practice and consider using TC more often — I’m just not ready to tell
patients that I know it’s a better regimen.
 DR DICKLER: I believe that it can be equal, but potentially less toxic, with the
doxorubicin eliminated.
DR DICKLER: I believe that it can be equal, but potentially less toxic, with the
doxorubicin eliminated.
 DR WINER: Absolutely.
DR WINER: Absolutely.
Tracks 17-22
 DR LOVE: Joe, how do you feel the Oncotype DX assay compares to other
assays? DR LOVE: Joe, how do you feel the Oncotype DX assay compares to other
assays? |
 DR SPARANO: The key difference with Oncotype DX is that it gives you three
categories as opposed to two. With most other genomic studies, you have a
good group and a bad group, but this study also indicates a midrange group.
For the patients whose recurrence score is very high or very low, you obtain
a definitively informative result. In practice, what’s happening is that more patients are falling into the midrange group, and that result doesn’t help you
make a clinical decision.
DR SPARANO: The key difference with Oncotype DX is that it gives you three
categories as opposed to two. With most other genomic studies, you have a
good group and a bad group, but this study also indicates a midrange group.
For the patients whose recurrence score is very high or very low, you obtain
a definitively informative result. In practice, what’s happening is that more patients are falling into the midrange group, and that result doesn’t help you
make a clinical decision.
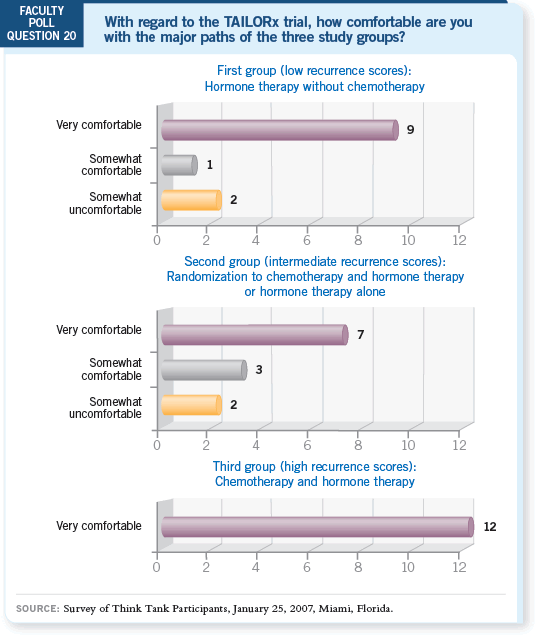
 DR LOVE: Aman, what’s your take on this?
DR LOVE: Aman, what’s your take on this?
 DR BUZDAR: The problem with the Oncotype DX data is that they are retrospective.
TAILORx is an important trial because it will determine whether
this assay is of value in identifying subsets of patients prospectively.
DR BUZDAR: The problem with the Oncotype DX data is that they are retrospective.
TAILORx is an important trial because it will determine whether
this assay is of value in identifying subsets of patients prospectively.
 DR WINER: I believe Oncotype DX is built on solid ground, albeit retrospective
in a prospectively defined cohort. I do use the Oncotype DX assay in practice, but not for all patients because I question how much value we can
obtain from chemotherapy for many patients with ER-positive, node-negative
breast cancer. While it doesn’t help me in every case, in some it does help push
me in one direction or another.
DR WINER: I believe Oncotype DX is built on solid ground, albeit retrospective
in a prospectively defined cohort. I do use the Oncotype DX assay in practice, but not for all patients because I question how much value we can
obtain from chemotherapy for many patients with ER-positive, node-negative
breast cancer. While it doesn’t help me in every case, in some it does help push
me in one direction or another.
 DR DICKLER: When we read a pathology report and consider the size, grade
and estrogen receptor status of the tumor, we’re putting patients on curves
using historical data. To me, Oncotype DX and Adjuvant! Online perform
the same function but in a different manner, and I believe it is the way of the
future.
DR DICKLER: When we read a pathology report and consider the size, grade
and estrogen receptor status of the tumor, we’re putting patients on curves
using historical data. To me, Oncotype DX and Adjuvant! Online perform
the same function but in a different manner, and I believe it is the way of the
future.
I do use Oncotype DX, and although I don’t use it all the time, for patients
with ER-positive disease, who I suspect gain little from chemotherapy, I feel it
helps me better stratify their risk.
 DR LEYLAND-JONES: Let me be provocative. If we can use the “poor man’s”
equivalent of the Oncotype DX assay and examine the tumor’s ER status, PR
status, grade and Ki67, then how do we justify the Oncotype DX?
DR LEYLAND-JONES: Let me be provocative. If we can use the “poor man’s”
equivalent of the Oncotype DX assay and examine the tumor’s ER status, PR
status, grade and Ki67, then how do we justify the Oncotype DX?
 DR SPARANO: I couldn’t agree more that you could possibly obtain the same
results by reliably examining ER, PR, grade and maybe Ki67, but the operative
word is “reliably.” All of these assays are notoriously difficult, and we see
great variation, perhaps less for ER and PR, but certainly for grade and Ki67.
DR SPARANO: I couldn’t agree more that you could possibly obtain the same
results by reliably examining ER, PR, grade and maybe Ki67, but the operative
word is “reliably.” All of these assays are notoriously difficult, and we see
great variation, perhaps less for ER and PR, but certainly for grade and Ki67.
 DR GOSS: My understanding is that grade is incorporated in the signature,
but an intraobserver variation appeared in the original assessment of grade
when the score was developed. In some published data, you can see pathologists
calling it Grade I versus Grade III, and vice versa. It’s not a lot of cases,
but it’s enough to influence the scoring system.
DR GOSS: My understanding is that grade is incorporated in the signature,
but an intraobserver variation appeared in the original assessment of grade
when the score was developed. In some published data, you can see pathologists
calling it Grade I versus Grade III, and vice versa. It’s not a lot of cases,
but it’s enough to influence the scoring system.
In our institution, investigators and pathologists continue to argue that
the Oncotype DX assay is a test to eliminate sloppy pathology reporting in
community oncology settings, whereas in a high-quality pathology setting, the
score doesn’t add that much value to the parameters we’ve been discussing.
 DR LOVE: Joe, do you think that’s the case?
DR LOVE: Joe, do you think that’s the case?
 DR SPARANO: That may be true, and the TAILORx trial may provide the
opportunity to test that hypothesis by evaluating the local versus central determination
of some of those factors in academic versus community centers.
DR SPARANO: That may be true, and the TAILORx trial may provide the
opportunity to test that hypothesis by evaluating the local versus central determination
of some of those factors in academic versus community centers.
 DR PEGRAM: The promise that Oncotype DX brings to the field is the application
of the multiplex PCR technology. It’s just a first step. It’s simply a 21-
gene set. It happens to be the proof-of-principle gene set for the technology,
and in the future this will probably be the least important gene set to examine
in breast cancer.
DR PEGRAM: The promise that Oncotype DX brings to the field is the application
of the multiplex PCR technology. It’s just a first step. It’s simply a 21-
gene set. It happens to be the proof-of-principle gene set for the technology,
and in the future this will probably be the least important gene set to examine
in breast cancer.
For example, a 186-gene set that’s a marker of stem cell populations was
recently published in The New England Journal of Medicine, with a discussion of
its impact on prognosis (Liu 2007). Suites of genes will likely emerge that are
predictive for response to individual targeted or cytotoxic agents and that will trump anything we gain from the current generation of multiplex PCR assays.
I believe that’s the direction in which the field needs to move.
 DR SPARANO: That’s exactly the point, and that’s why a coprimary objective
of the TAILORx trial is to collect these tissues and peripheral blood specimens
that we can use to evaluate other diagnostic tests as the technology emerges in
the next five to 10 years.
DR SPARANO: That’s exactly the point, and that’s why a coprimary objective
of the TAILORx trial is to collect these tissues and peripheral blood specimens
that we can use to evaluate other diagnostic tests as the technology emerges in
the next five to 10 years.
 DR ELLIS: I believe all those who think that a “poor man’s” Oncotype DX assay is out there are deluding themselves. I was in the room when the
PACCT-1 trial was being designed and, thinking we could perhaps randomly
assign the intermediate group based on several risk strata, we reviewed all the
literature on Ki67, grading, ploidy and all the other factors that have been
examined, and it was clear that precision in those assays was totally inadequate
for designing a prospective trial.
DR ELLIS: I believe all those who think that a “poor man’s” Oncotype DX assay is out there are deluding themselves. I was in the room when the
PACCT-1 trial was being designed and, thinking we could perhaps randomly
assign the intermediate group based on several risk strata, we reviewed all the
literature on Ki67, grading, ploidy and all the other factors that have been
examined, and it was clear that precision in those assays was totally inadequate
for designing a prospective trial.
Fifteen years of attempts to create a Ki67 assay have failed, and this continues
to be a big problem. I use that assay in my lab, but only with regard to biological
endpoints for research. As a clinical assay, it’s a nightmare.
What quantitative PCR brought to the table was precision. The patient in
front of you has a binary outcome — either she will relapse or she won’t
— and we’re trying to predict that outcome. That individual doesn’t have a
gray zone of risk. So, if you consider that risk score, I believe you can identify
patients — who are actually at the lowest-risk end — whom you can tell with
about 95 percent confidence, “You’re not going to relapse from your breast
cancer.” I believe that’s a powerful statement.
My point is that the TAILORx trial includes an observational cohort, which is
the low-risk group, for whom we will confirm prospectively that they’re truly
at low risk. For me, to be able to define that group is comforting and a big
step forward.
 DR WINER: The TAILORx trial will generate a rich data set. I don’t believe
anyone is suggesting that Oncotype DX is the be-all and end-all and that
something better won’t emerge. I would tend to agree that in expert centers
where the pathology is particularly good and testing for ER, PR and HER2 is
particularly reliable, tests like this are less useful.
DR WINER: The TAILORx trial will generate a rich data set. I don’t believe
anyone is suggesting that Oncotype DX is the be-all and end-all and that
something better won’t emerge. I would tend to agree that in expert centers
where the pathology is particularly good and testing for ER, PR and HER2 is
particularly reliable, tests like this are less useful.
However, Paul and I live in the same town, and if we have our expert breast
pathologists examine the same specimen, most of the time they agree but
not all the time. In addition, not all of our breast specimens are read at either
center by the expert breast pathologists, and once you move outside of that
select group, you hear a fair amount of disagreement about grade.
 DR SPARANO: I have one final comment about the midrange score for the
listening clinicians. If you examine the risk of distant recurrence for patients
with a midrange recurrence score, you see that the 10-year distant relapse-free
interval was 95 percent for patients treated with tamoxifen and 94 percent for
those treated with tamoxifen and chemotherapy (Paik 2006; Sparano 2006).
DR SPARANO: I have one final comment about the midrange score for the
listening clinicians. If you examine the risk of distant recurrence for patients
with a midrange recurrence score, you see that the 10-year distant relapse-free
interval was 95 percent for patients treated with tamoxifen and 94 percent for
those treated with tamoxifen and chemotherapy (Paik 2006; Sparano 2006).
For those who are concerned about randomly assigning patients with a score
in that range, those data should help them feel more comfortable.
Select Publications

