
| Tracks 1-22 |
| Track 1 |
Paclitaxel and bevacizumab for
patients who have previously
received adjuvant taxane therapy |
| Track 2 |
Potential benefit of combining
bevacizumab and capecitabine |
| Track 3 |
Tolerability and efficacy of
paclitaxel and bevacizumab as
first-line therapy for metastatic
disease |
| Track 4 |
Potential rationale for the slow
incorporation of bevacizumab into
clinical practice |
| Track 5 |
Weighing the costs of therapy
versus the benefit to patients |
| Track 6 |
Rationale for using bevacizumab
beyond the first-line setting |
| Track 7 |
Need for an ongoing dialogue
about the rising cost of therapies |
| Track 8 |
Trend for improvement in survival
in ECOG-E2100 |
| Track 9 |
Clinical use of bevacizumab in
combination with capecitabine |
| Track 10 |
Continuation of bevacizumab
after disease progression |
| Track 11 |
XCaliBr: Phase II study of
capecitabine with bevacizumab
followed by bevacizumab upon
progression |
|
| Track 12 |
Mechanisms of resistance to anti-angiogenic
therapy |
| Track 13 |
Importance of weighing overall
societal costs versus the cost of
an individual therapy |
| Track 14 |
Potential biologic rationale for
benefit of adjuvant bevacizumab |
| Track 15 |
Evaluating the optimal duration of
adjuvant bevacizumab |
| Track 16 |
Potential benefit of fulvestrant
in combination with aromatase
inhibitors |
| Track 17 |
Ovarian suppression and fulvestrant
for premenopausal women |
| Track 18 |
Biologic rationale for using a
loading dose of fulvestrant |
| Track 19 |
Sequencing hormonal therapy for
premenopausal women with ER-positive
metastatic disease |
| Track 20 |
Influence of aromatase inhibitors
on intratumoral estrogen levels |
| Track 21 |
Incorporation of fulvestrant into
the adjuvant setting |
| Track 22 |
Potential role of fulvestrant
after five years of an aromatase
inhibitor |
|
|
Select Excerpts from the Discussion
 Tracks 1-2
Tracks 1-2
 DR LOVE: Dr Miller, would you comment on ECOG-E2100 and the
treatment of patients who previously received an adjuvant taxane? DR LOVE: Dr Miller, would you comment on ECOG-E2100 and the
treatment of patients who previously received an adjuvant taxane? |
 DR MILLER: This trial (Miller 2005a) specifically allowed patients who had
had an adjuvant taxane as long as their disease-free interval was greater than
12 months. Approximately 18 percent of the patients were in this situation, and they were nicely matched between the two treatment groups.
DR MILLER: This trial (Miller 2005a) specifically allowed patients who had
had an adjuvant taxane as long as their disease-free interval was greater than
12 months. Approximately 18 percent of the patients were in this situation, and they were nicely matched between the two treatment groups.
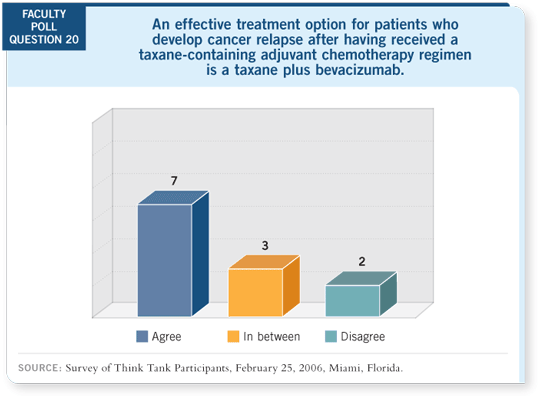
The overall result in these patients was essentially a doubling of objective
response rates, which translated into a highly significant, more than five-month
improvement in progression-free survival. It’s certainly fair to wonder
if those results held up in the patients who received taxane-containing
adjuvant therapy.
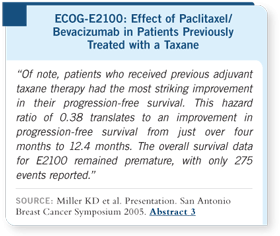 We have evaluated a variety
of subsets, including the
subset that received previous
taxane-based therapy. Their
hazard ratio was 0.38,
compared to 0.51 for the
overall group. This translated
into an improvement in their
progression-free survival
from four months to 12.4
months. So, if you’ve had an
adjuvant taxane, you do gain
substantial benefit from a
taxane plus bevacizumab.
We have evaluated a variety
of subsets, including the
subset that received previous
taxane-based therapy. Their
hazard ratio was 0.38,
compared to 0.51 for the
overall group. This translated
into an improvement in their
progression-free survival
from four months to 12.4
months. So, if you’ve had an
adjuvant taxane, you do gain
substantial benefit from a
taxane plus bevacizumab.
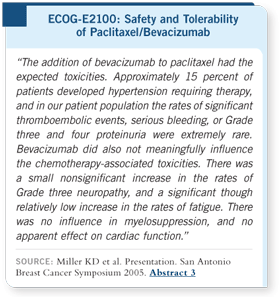
Toxicity in this trial was also favorable, with 15 to 16 percent of patients developing hypertension that
needed therapy and no major differences in the chemotherapy-related toxicities.
There were slight increases in fatigue and neuropathy, likely because
patients were responding for longer durations, so they received more exposure
to chemotherapy.
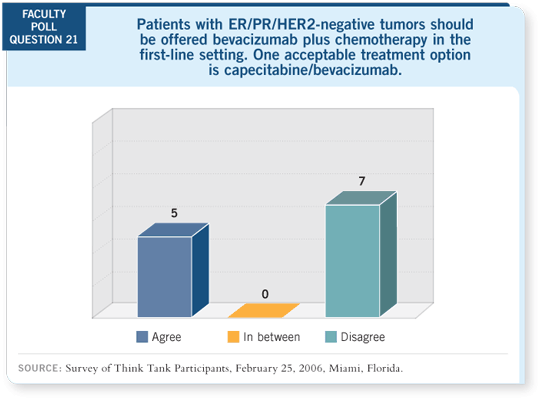
In whom would I not consider this combination? One obvious group is
patients who were not eligible for the E2100 trial, who received an adjuvant
taxane and relapsed in fewer than 12 months. Those patients actually were
allowed to enroll in the previous randomized trial of capecitabine with or
without bevacizumab as their first therapy (Miller 2005b).
This previous trial also found increases in response rate by adding bevacizumab
to capecitabine but no difference in progression-free survival. There
were, however, huge differences in the patient populations, particularly in
the extent of previous chemotherapy. About a third of patients in E2100 were
completely chemotherapy-naïve, including no adjuvant chemotherapy and
much less exposure to previous taxanes.
I believe the biggest difference between the trials is a matter of timing — as
breast cancers progress, they express a greater number and have greater redundancy
in the proangiogenic pathways. This explanation fits the E2100 data
nicely and is why, for this patient, I would strongly recommend a taxane
and bevacizumab-containing regimen rather than some other chemotherapy combination and holding
bevacizumab in reserve until
further progression.
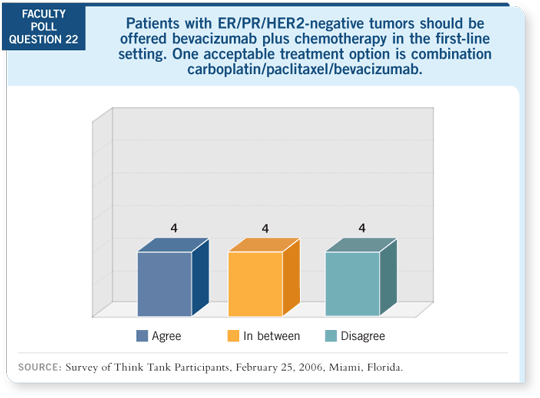
 Tracks 3-5
Tracks 3-5
 DR LOVE: Many oncologists
tell us they’re
confused about where
bevacizumab fits into the
management of metastatic
breast cancer. Does that
surprise you, Kathy? DR LOVE: Many oncologists
tell us they’re
confused about where
bevacizumab fits into the
management of metastatic
breast cancer. Does that
surprise you, Kathy? |
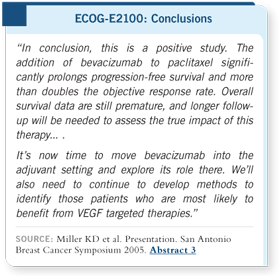
 DR MILLER: It has surprised
me from the day I presented
these results. No other trial
in first-line treatment for
metastatic breast cancer has found this degree of improvement in outcome
with this minimal toxicity (Miller 2005a). The only study that has come close
is the original trastuzumab randomized trial (Slamon 2001), and that was
hampered by a significant rate of congestive heart failure in one of the two arms.
DR MILLER: It has surprised
me from the day I presented
these results. No other trial
in first-line treatment for
metastatic breast cancer has found this degree of improvement in outcome
with this minimal toxicity (Miller 2005a). The only study that has come close
is the original trastuzumab randomized trial (Slamon 2001), and that was
hampered by a significant rate of congestive heart failure in one of the two arms.
I don’t recall anyone looking at those results and saying, “It’s only five months
and we just don’t know
where this fits in. We have
so many other things to give
patients.”
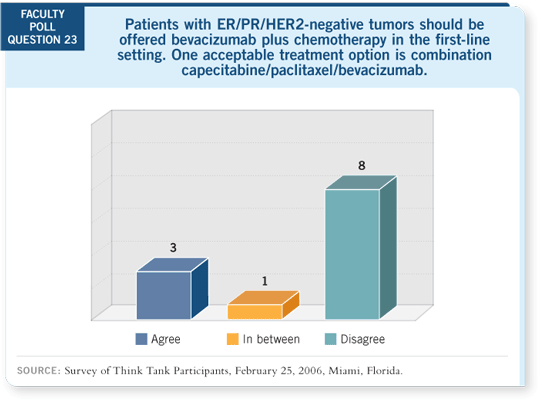
 No other drugs were
approved for breast cancer
between then and the E2100
data. And this trial applies
to a much larger subset of
our patients. I quite honestly
don’t understand the reluctance.
No other drugs were
approved for breast cancer
between then and the E2100
data. And this trial applies
to a much larger subset of
our patients. I quite honestly
don’t understand the reluctance.
 DR BURSTEIN: I like the
E2100 study, and it’s an
exciting proof of principle.
I would use the regimen for
treatment of the patient who
had received an adjuvant
taxane, but let me ask you to play it out a little more. For instance, we have trials of combination versus
sequential chemotherapy with a statistically significant survival advantage. I
have, in general, resisted those, believing that we should treat sequentially.
DR BURSTEIN: I like the
E2100 study, and it’s an
exciting proof of principle.
I would use the regimen for
treatment of the patient who
had received an adjuvant
taxane, but let me ask you to play it out a little more. For instance, we have trials of combination versus
sequential chemotherapy with a statistically significant survival advantage. I
have, in general, resisted those, believing that we should treat sequentially.
One of the challenges your two trials pose for me is that they suggest a
relatively specific window during which bevacizumab is effective. Conceptually,
I find it hard to imagine that the drug works in the first-line setting but
not elsewhere.
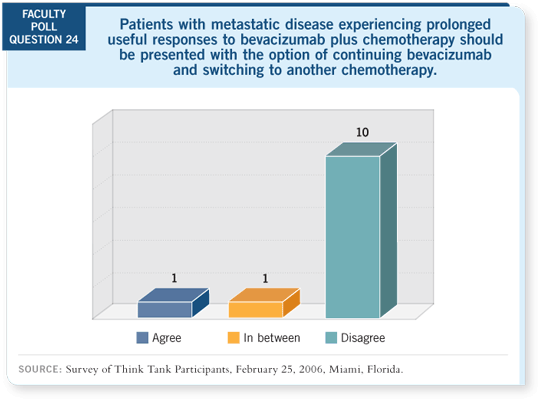
 DR MILLER: It may be hard to explain, but these are the data we have, and it
does fit the biology. We also have data suggesting that increases in proangiogenic
peptide expression result in a relative resistance to chemotherapy. These
become more numerous as patients progress, and this makes inhibiting any
single factor inherently less effective, which is different from the chemotherapy
trials.
DR MILLER: It may be hard to explain, but these are the data we have, and it
does fit the biology. We also have data suggesting that increases in proangiogenic
peptide expression result in a relative resistance to chemotherapy. These
become more numerous as patients progress, and this makes inhibiting any
single factor inherently less effective, which is different from the chemotherapy
trials.
The other difference is that the chemotherapy trials usually have only found
a progression-free survival improvement of two to three months at the cost
of substantial increases in toxicity. I suspect we will have an overall survival
improvement; it’s just too early to know yet.
This should not be taken as an assumption that no survival advantage exists,
merely that it’s an effective therapy. We have to wait longer to get those
results.
 DR WINER: I, too, am enthusiastic about bevacizumab, but three issues have
led people to be less enthusiastic. One is that this applies to a large subset
of patients. I believe people would be happier if this could be targeted to a
specific group. People are also less enthusiastic because of not knowing quite
what to do with the results of the capecitabine trial. The third and very real
issue is the cost.
DR WINER: I, too, am enthusiastic about bevacizumab, but three issues have
led people to be less enthusiastic. One is that this applies to a large subset
of patients. I believe people would be happier if this could be targeted to a
specific group. People are also less enthusiastic because of not knowing quite
what to do with the results of the capecitabine trial. The third and very real
issue is the cost.
 DR HUDIS: Unfortunately, the cost got in everyone’s way. This is the first
drug that forced a change in dispensing practices for our whole institution. We
can’t write for it without pre-approval from the insurance company, and we’ve
never had that for any agent in our setting.
DR HUDIS: Unfortunately, the cost got in everyone’s way. This is the first
drug that forced a change in dispensing practices for our whole institution. We
can’t write for it without pre-approval from the insurance company, and we’ve
never had that for any agent in our setting.
The second issue, which we don’t talk much about, is that although the
toxicities are manageable and those of us who used the drug got used to it, it
represents a little bit of a change in practice patterns for oncologists. They’re
suddenly paying attention to proteinuria and hypertension.
 DR MILLER: I don’t deny that the cost is an issue. But the cost is not
markedly different from the cost of trastuzumab when it was first available,
and I don’t recall reluctance with that agent.
DR MILLER: I don’t deny that the cost is an issue. But the cost is not
markedly different from the cost of trastuzumab when it was first available,
and I don’t recall reluctance with that agent.
When I’ve heard people talk about their reluctance, they haven’t said, “If cost
were not an issue, I would use it in a heartbeat.” So I think cost is one component
of the reluctance but certainly not the only one.
 DR SLEDGE: Physicians like to be able to say, “This drug will improve your
survival by X months.” I think part of the problem with this drug is that we
don’t have those survival data yet.
DR SLEDGE: Physicians like to be able to say, “This drug will improve your
survival by X months.” I think part of the problem with this drug is that we
don’t have those survival data yet.
From a quality-of-life standpoint, those of us who have used it have found it
to be an incredibly easy drug for patients, with truly trivial toxicity compared
to every single chemotherapeutic agent in the therapeutic armamentarium. It
also more than doubles the response rate.
 DR OSBORNE: I believe the cost of this drug has perhaps crossed the line in
the eyes of private practitioners. We’re beginning to realize there’s a limit.
DR OSBORNE: I believe the cost of this drug has perhaps crossed the line in
the eyes of private practitioners. We’re beginning to realize there’s a limit.
 Track 5
Track 5
 DR LOVE: Kathy, what has been your reaction to the discussions
regarding the cost of bevacizumab? DR LOVE: Kathy, what has been your reaction to the discussions
regarding the cost of bevacizumab? |
 DR MILLER: I am frustrated by the inconsistency in how we view costs of
therapies. In many settings we routinely use growth factors and expensive
supportive care agents for regimens that have a low risk, when the guidelines
wouldn’t suggest it, and people order lots of horrendously expensive combined
PET/CT scans, which don’t add to treatment.
DR MILLER: I am frustrated by the inconsistency in how we view costs of
therapies. In many settings we routinely use growth factors and expensive
supportive care agents for regimens that have a low risk, when the guidelines
wouldn’t suggest it, and people order lots of horrendously expensive combined
PET/CT scans, which don’t add to treatment.
So I have a problem hearing about the cost of one specific drug that had a huge benefit in this trial. I’m not arguing that we shouldn’t consider the costs. Of course, they’re important for all of our practices, our individual patients
and our society. But to consider the costs in a vacuum only as they apply to
one drug is a mistake.
 Tracks 6-7
Tracks 6-7
 DR LOVE: Cliff, are you using capecitabine combined with
bevacizumab? DR LOVE: Cliff, are you using capecitabine combined with
bevacizumab? |
 DR HUDIS: Absolutely. The data are not really negative (Miller 2005b). The
response rate is higher. A principle has been clearly established, in my mind,
that bevacizumab adds to chemotherapy in a cohort of patients.
DR HUDIS: Absolutely. The data are not really negative (Miller 2005b). The
response rate is higher. A principle has been clearly established, in my mind,
that bevacizumab adds to chemotherapy in a cohort of patients.
 DR BURSTEIN: We should be in dialogue about where and how best to use
these therapies, and I take Kathy’s point that the expense is not unique to this
drug. There is a compelling reason to think we often overtreat in the way
of PET scans, stereotactic radiosurgery the third or fourth time around for
brain metastases, or unbelievable efforts at other supportive care, which have
a relatively modest cost-effective gain. I believe we should engage in a serious
dialogue about these issues.
DR BURSTEIN: We should be in dialogue about where and how best to use
these therapies, and I take Kathy’s point that the expense is not unique to this
drug. There is a compelling reason to think we often overtreat in the way
of PET scans, stereotactic radiosurgery the third or fourth time around for
brain metastases, or unbelievable efforts at other supportive care, which have
a relatively modest cost-effective gain. I believe we should engage in a serious
dialogue about these issues.
 Tracks 11-12
Tracks 11-12
 DR LOVE: George, what efforts are being made to determine the role of
bevacizumab with other agents in the first- and second-line settings? DR LOVE: George, what efforts are being made to determine the role of
bevacizumab with other agents in the first- and second-line settings? |
 DR SLEDGE: The XCaliBr trial uses front-line capecitabine with bevacizumab
for patients who have received basically any adjuvant chemotherapy.
DR SLEDGE: The XCaliBr trial uses front-line capecitabine with bevacizumab
for patients who have received basically any adjuvant chemotherapy.
This trial has recently been expanded to approximately 112 patients, and it
should have decent confidence intervals for response rate and progression-free
survival.
This trial also recommends that patients cross over to a second-line chemotherapy,
either vinorelbine or paclitaxel, at the investigator and patient’s
choice, with bevacizumab.
So we will obtain data from this trial in terms of second-line responses to
either vinorelbine or paclitaxel with bevacizumab. The data should be available
to us some time next year.
Resistance remains a big issue for anti-angiogenic therapy in just about every
disease that we’ve evaluated it in to date, and it’s certainly not surprising that it
will continue to be a problem.
Therefore, it’s not surprising that crossing over to another chemotherapy agent
with bevacizumab is unlikely to make much difference.
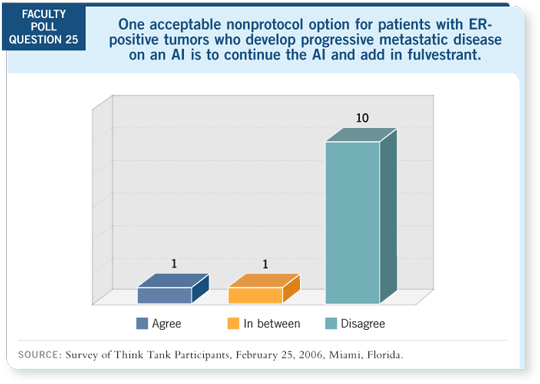
 Track 16
Track 16
 DR LOVE: John, do we have any information about combination
hormonal therapy with fulvestrant and an aromatase inhibitor in the
metastatic setting? DR LOVE: John, do we have any information about combination
hormonal therapy with fulvestrant and an aromatase inhibitor in the
metastatic setting? |
 DR ROBERTSON: This is being evaluated in the ongoing SoFEA trial, in
which the aromatase inhibitor is continued and fulvestrant is added on in the
hope that by keeping down the estradiol level, more fulvestrant will compete
with the receptor and perhaps give a better initial response or even longer-term
control. But I’m not sure that we’re going to see the result of this study
in our lifetime.
DR ROBERTSON: This is being evaluated in the ongoing SoFEA trial, in
which the aromatase inhibitor is continued and fulvestrant is added on in the
hope that by keeping down the estradiol level, more fulvestrant will compete
with the receptor and perhaps give a better initial response or even longer-term
control. But I’m not sure that we’re going to see the result of this study
in our lifetime.
Theoretically, fulvestrant with an aromatase inhibitor is as good as any other
option. The problem with this combined approach is that we have no human
data for any combination. We have nothing to suggest that this combination
will be better.
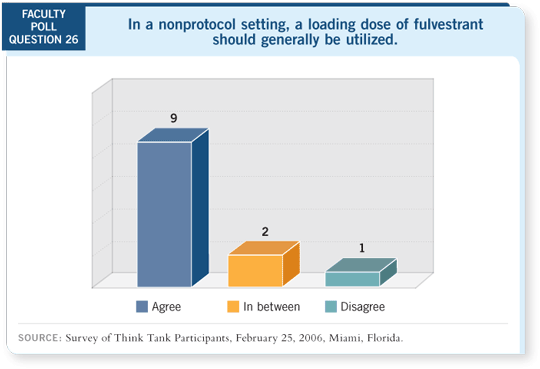
 Track 18
Track 18
 DR LOVE: What’s the evidence supporting a loading dose of fulvestrant? DR LOVE: What’s the evidence supporting a loading dose of fulvestrant? |
 DR ROBERTSON: First, tamoxifen reaches a steady state at two weeks,
whereas fulvestrant can take up to four or five months to reach a steady state.
DR ROBERTSON: First, tamoxifen reaches a steady state at two weeks,
whereas fulvestrant can take up to four or five months to reach a steady state.
Another issue, which I believe makes people slightly uncomfortable, is that in
the second-line study, fulvestrant was just as good as anastrozole after tamoxifen
(Howell 2002; Osborne 2002).
The first-line study, however, had two problems. Although it was a randomized
study, 10 percent more people were assigned to fulvestrant versus tamoxifen.
In addition, in the intention-to-treat population, the time-to-progression
curve for the initial fulvestrant arm drops down much more quickly than the
curve for tamoxifen, and then, after the first six months, it runs parallel to
tamoxifen. It makes one think that perhaps the drug is not on board in that
first six months.
The question is: why would you see this in the first-line and not the second-line
setting? You could argue that some of those patients in the second-line
setting may be having a tamoxifen withdrawal effect while the fulvestrant
levels are going up.
 DR HAYES: I would argue that this drug clearly has a dose-response curve.
Kent’s trials demonstrated that the lower dose had to be dropped because it
was ineffective (Osborne 2002).
DR HAYES: I would argue that this drug clearly has a dose-response curve.
Kent’s trials demonstrated that the lower dose had to be dropped because it
was ineffective (Osborne 2002).
In addition, I don’t know any drug we use for which we don’t want to use the
right dose. It’s clear from the pharmacokinetics of this drug that if you use the
loading dose, you reach what should be acceptable levels faster. We don’t use a
loading dose for tamoxifen because patients take it every day.
 Track 21
Track 21
 DR LOVE: Hal, what investigational strategies are being pursued with
fulvestrant? DR LOVE: Hal, what investigational strategies are being pursued with
fulvestrant? |
 DR BURSTEIN: We have a wealth of endocrine options coming forward.
How to integrate fulvestrant is one of them. Many of us are starting to think
about it in the adjuvant setting.
DR BURSTEIN: We have a wealth of endocrine options coming forward.
How to integrate fulvestrant is one of them. Many of us are starting to think
about it in the adjuvant setting.
What’s disappointing is that we don’t really have a surrogate, short of a large,
randomized, prospective study that will take a decade to finish, to tell us what
to do with this drug.
It’s at the fundamental level of failure of what our laboratory correlative
studies have allowed us to do so far because we’re still left having to resort
to tremendously large studies to answer these questions. It’s a real barrier for
more rapid integration.
The question is: fulvestrant with or without an aromatase inhibitor or fulvestrant
after an aromatase inhibitor in the adjuvant setting or combinations
thereof ?
 Track 22
Track 22
 DR LOVE: Eric, can you talk about the delayed fulvestrant trial? DR LOVE: Eric, can you talk about the delayed fulvestrant trial? |
 DR WINER: This is not fully hashed out by any means.
DR WINER: This is not fully hashed out by any means.
We’ve prepared a concept of a trial looking at fulvestrant in the extended
adjuvant setting for women who have received five years of an aromatase
inhibitor or who have received tamoxifen followed by some amount of an
aromatase inhibitor.
The concept would be to compare fulvestrant with either no therapy or a
placebo in those women and potentially allow women to start on the therapy
even after a break of a year or two or three years, with the idea that whenever
a woman with ER-positive breast cancer starts a new endocrine therapy, a
benefit and a decrease in events may occur.
Select publications

